Heat Q Definition . is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. enthalpy and heat are entirely different things. Calculate and interpret heat and related properties. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Another unit is the calorie. If you know the state of a system,. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The standard unit of heat in the international system of units (si) is the joule (j). Explain the technique of calorimetry. the quantitative relationship between heat transfer and temperature change contains all three factors: \[q = mc\delta t,\] where \(q\) is the symbol for. Is defined through the formula q =. Enthalpy is a function of state. the usual symbol for heat is ‘q’.
from gamesmartz.com
Calculate and interpret heat and related properties. Enthalpy is a function of state. is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. enthalpy and heat are entirely different things. the usual symbol for heat is ‘q’. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. \[q = mc\delta t,\] where \(q\) is the symbol for. If you know the state of a system,. The standard unit of heat in the international system of units (si) is the joule (j).
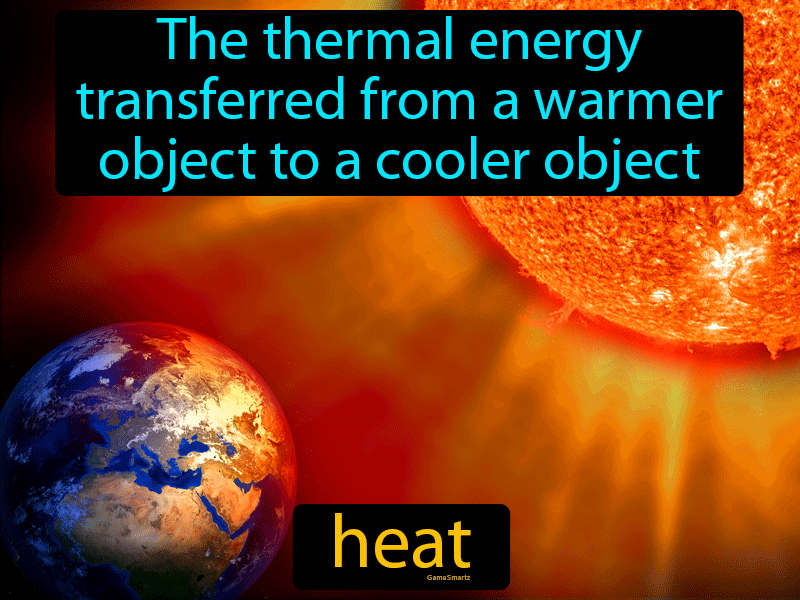
Heat Easy to Understand Definition
Heat Q Definition enthalpy and heat are entirely different things. Is defined through the formula q =. Calculate and interpret heat and related properties. If you know the state of a system,. is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. \[q = mc\delta t,\] where \(q\) is the symbol for. enthalpy and heat are entirely different things. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. the quantitative relationship between heat transfer and temperature change contains all three factors: Explain the technique of calorimetry. Enthalpy is a function of state. The standard unit of heat in the international system of units (si) is the joule (j). Another unit is the calorie. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the usual symbol for heat is ‘q’.
From gamesmartz.com
Heat Easy to Understand Definition Heat Q Definition Explain the technique of calorimetry. Enthalpy is a function of state. Another unit is the calorie. If you know the state of a system,. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. the quantitative relationship between heat transfer and temperature change contains all three factors: . Heat Q Definition.
From www.britannica.com
Conductivity physics Britannica Heat Q Definition Enthalpy is a function of state. Is defined through the formula q =. the usual symbol for heat is ‘q’. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Explain the technique of calorimetry. Calculate and interpret heat and related properties. is a measure of the. Heat Q Definition.
From slideplayer.com
CHEM 3310 Thermodynamics. ppt download Heat Q Definition the usual symbol for heat is ‘q’. is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. enthalpy and heat are entirely different things. \[q = mc\delta t,\] where \(q\) is the symbol for. If you know the state of a system,. Explain the technique of calorimetry. Another unit. Heat Q Definition.
From slideplayer.com
Heat, q energy that transfers from one object to another, because of a Heat Q Definition q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Explain the technique of calorimetry. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. the. Heat Q Definition.
From slideplayer.com
Reaction Energy. ppt download Heat Q Definition Another unit is the calorie. \[q = mc\delta t,\] where \(q\) is the symbol for. enthalpy and heat are entirely different things. Explain the technique of calorimetry. If you know the state of a system,. is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. Enthalpy is a function of. Heat Q Definition.
From slideplayer.com
Thermochemistry. ppt download Heat Q Definition is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the heat capacity of a substance describes how its temperature changes. Heat Q Definition.
From www.chegg.com
Solved Define heat capacity and specific heat capacity of a Heat Q Definition the usual symbol for heat is ‘q’. Another unit is the calorie. The standard unit of heat in the international system of units (si) is the joule (j). Explain the technique of calorimetry. \[q = mc\delta t,\] where \(q\) is the symbol for. the heat capacity of a substance describes how its temperature changes as it absorbs or. Heat Q Definition.
From www.youtube.com
What is Heat Heat Definition Physics Heat Energy in Hindi, Urdu Heat Q Definition the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. If you know the state of a system,. Explain the technique of calorimetry. the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol. Heat Q Definition.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download Heat Q Definition Explain the technique of calorimetry. Is defined through the formula q =. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. the quantitative relationship between heat transfer and temperature change contains all three factors: If you know the state of a system,. Calculate and interpret heat and. Heat Q Definition.
From slideplayer.com
CALORIMETRY Calorimetry measurement of heat changes ppt download Heat Q Definition \[q = mc\delta t,\] where \(q\) is the symbol for. enthalpy and heat are entirely different things. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. the usual symbol for heat is ‘q’. is a measure of the heat energy (q) per mass (m) released. Heat Q Definition.
From slideplayer.com
CALORIMETRY Calorimetry measurement of heat changes ppt download Heat Q Definition enthalpy and heat are entirely different things. is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Explain the technique of calorimetry. The standard unit of heat in the. Heat Q Definition.
From study.com
Latent Heat Definition, Formula & Examples Video & Lesson Transcript Heat Q Definition the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. the usual symbol for heat is ‘q’. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. \[q = mc\delta t,\] where \(q\) is. Heat Q Definition.
From slideplayer.com
Thermochemistry The study of heat changes that occur during chemical Heat Q Definition Explain the technique of calorimetry. the usual symbol for heat is ‘q’. \[q = mc\delta t,\] where \(q\) is the symbol for. The standard unit of heat in the international system of units (si) is the joule (j). If you know the state of a system,. is a measure of the heat energy (q) per mass (m) released. Heat Q Definition.
From ch301.cm.utexas.edu
heating curve Heat Q Definition enthalpy and heat are entirely different things. Explain the technique of calorimetry. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Enthalpy is a function of state. Another unit is the calorie. the heat capacity formula is expressed as the product of mass,. Heat Q Definition.
From slideplayer.com
THERMOCHEMISTRY or Thermodynamics ppt download Heat Q Definition q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Is defined through the formula q =. Another unit is the calorie. the usual symbol for heat is ‘q’. Calculate and interpret heat and related properties. the heat capacity. Heat Q Definition.
From www.researchgate.net
Heat capacity C and latent heat Q in the range of transition between Heat Q Definition the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Enthalpy is a function of state. Explain the technique of calorimetry. Another unit is the calorie. The standard unit of heat in the international system of units (si) is the joule (j). q = mcδt, where q is. Heat Q Definition.
From www.grc.nasa.gov
Entropy of a Gas Heat Q Definition q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calculate and interpret heat and related properties. Explain the technique of calorimetry. the usual symbol for heat is ‘q’. Another unit is the calorie. the heat capacity of a. Heat Q Definition.
From www.slideserve.com
PPT Chapter 20 Heat, specific heat Internal energy First law of Heat Q Definition the usual symbol for heat is ‘q’. Another unit is the calorie. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Is defined through the formula q =. the heat capacity formula is expressed as the product of. Heat Q Definition.
From www.youtube.com
Derivation of Heating effect of electric current Joule's Law of Heat Q Definition Another unit is the calorie. Explain the technique of calorimetry. If you know the state of a system,. enthalpy and heat are entirely different things. Is defined through the formula q =. The standard unit of heat in the international system of units (si) is the joule (j). the heat capacity formula is expressed as the product of. Heat Q Definition.
From qdotsystems.com.au
A Derivation of the Heat Conduction Equation Heat Q Definition The standard unit of heat in the international system of units (si) is the joule (j). is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. Enthalpy is a function of state. Another unit is the calorie. Is defined through the formula q =. Explain the technique of calorimetry. the. Heat Q Definition.
From www.youtube.com
How to Solve for Heat (Q) Released in a Reaction using Q=mcT Equation Heat Q Definition Enthalpy is a function of state. Another unit is the calorie. Is defined through the formula q =. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the usual symbol for heat is ‘q’. The standard unit of heat. Heat Q Definition.
From studylib.net
Heat Equation Heat Q Definition is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. \[q = mc\delta t,\] where \(q\) is the symbol for. enthalpy and heat are entirely different things. the usual symbol for heat is ‘q’. the quantitative relationship between heat transfer and temperature change contains all three factors: . Heat Q Definition.
From thermalengineeringlearn.blogspot.com
THERMAL ENGINEERING Thermodynamic Equilibrium Heat Q Definition \[q = mc\delta t,\] where \(q\) is the symbol for. the usual symbol for heat is ‘q’. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calculate and interpret heat and related properties. the quantitative relationship between heat. Heat Q Definition.
From slideplayer.com
Amount of heat transferred = mass x change in temperature x specific Heat Q Definition Explain the technique of calorimetry. Another unit is the calorie. the quantitative relationship between heat transfer and temperature change contains all three factors: the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Is defined through the formula q =. If you know the state of a system,.. Heat Q Definition.
From askfilo.com
When heat Q is supplied to one mole of monoatomic gas undergoing a thermo.. Heat Q Definition q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The standard unit of heat in the international system of units (si) is the joule (j). \[q = mc\delta t,\] where \(q\) is the symbol for. the usual symbol for. Heat Q Definition.
From ar.inspiredpencil.com
Heat Definition Heat Q Definition the quantitative relationship between heat transfer and temperature change contains all three factors: enthalpy and heat are entirely different things. \[q = mc\delta t,\] where \(q\) is the symbol for. the usual symbol for heat is ‘q’. Is defined through the formula q =. Explain the technique of calorimetry. Enthalpy is a function of state. The standard. Heat Q Definition.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free Heat Q Definition The standard unit of heat in the international system of units (si) is the joule (j). the quantitative relationship between heat transfer and temperature change contains all three factors: Is defined through the formula q =. If you know the state of a system,. the heat capacity formula is expressed as the product of mass, specific heat, and. Heat Q Definition.
From www.thoughtco.com
Definition and Examples of Heat Energy Heat Q Definition The standard unit of heat in the international system of units (si) is the joule (j). Calculate and interpret heat and related properties. enthalpy and heat are entirely different things. Another unit is the calorie. \[q = mc\delta t,\] where \(q\) is the symbol for. the usual symbol for heat is ‘q’. the quantitative relationship between heat. Heat Q Definition.
From www.slideserve.com
PPT Quantifying Heat PowerPoint Presentation, free download ID2579013 Heat Q Definition Calculate and interpret heat and related properties. Explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the usual symbol for heat is ‘q’. the heat capacity of a substance describes how its temperature. Heat Q Definition.
From www.slideserve.com
PPT An introduction to Thermodynamics PowerPoint Presentation, free Heat Q Definition is a measure of the heat energy (q) per mass (m) released or absorbed during a phase change. The standard unit of heat in the international system of units (si) is the joule (j). Explain the technique of calorimetry. Enthalpy is a function of state. q = mcδt, where q is the symbol for heat transfer (“quantity of. Heat Q Definition.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Q Definition Is defined through the formula q =. the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the usual symbol for heat is ‘q’. Enthalpy is. Heat Q Definition.
From www.pinterest.com
Specific Heat Easy Science Teaching chemistry, Sensible heat Heat Q Definition q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Is defined through the formula q =. If you know the state of a system,. is a measure of the heat energy (q) per mass (m) released or absorbed during. Heat Q Definition.
From giozigmtv.blob.core.windows.net
Define Heat Transfer at June McElhannon blog Heat Q Definition the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Calculate and interpret heat and related properties. If you know the state of a system,. the quantitative relationship between heat transfer and temperature change contains all three factors: \[q = mc\delta t,\] where \(q\) is. Heat Q Definition.
From www.numerade.com
SOLVED In order to calculate the amount of heat (q) that flows into or Heat Q Definition If you know the state of a system,. \[q = mc\delta t,\] where \(q\) is the symbol for. Is defined through the formula q =. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Calculate and interpret heat and related properties. Another unit is the. Heat Q Definition.
From getrevising.co.uk
Physicsend of year 9 Revision Cards in GCSE Physics Heat Q Definition the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Explain the technique of calorimetry. The standard unit of heat in the international system of units (si) is the joule (j). If you know the state of a system,. the usual symbol for heat is ‘q’. is. Heat Q Definition.