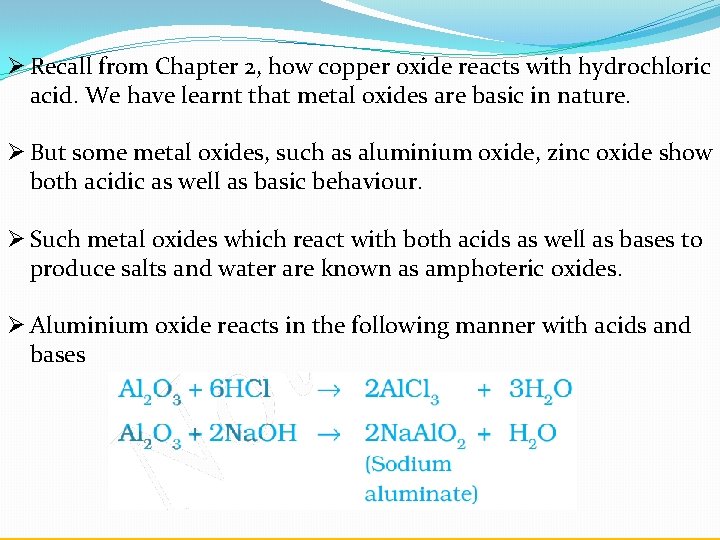
Copper Oxide Reacts With Hydrochloric Acid . this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo + 2hcl → cucl2. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. cucl_2(aq) and the reaction. The substances used are copper oxide and dilute. this video demonstrates the action of acids on metal oxides. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l.
from slidetodoc.com
copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo + 2hcl → cucl2. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. cucl_2(aq) and the reaction. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. The substances used are copper oxide and dilute. this video demonstrates the action of acids on metal oxides.
SSLC ONLINE CLASSES Grade 10 Chapter 3 Subject
Copper Oxide Reacts With Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. The substances used are copper oxide and dilute. cucl_2(aq) and the reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. this video demonstrates the action of acids on metal oxides. Cuo + 2hcl → cucl2.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Copper Oxide Reacts With Hydrochloric Acid Cuo + 2hcl → cucl2. this video demonstrates the action of acids on metal oxides. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. cucl_2(aq) and the reaction. The substances used are copper oxide and dilute. this reaction is a type of double replacement reaction called a neutralization reaction,. Copper Oxide Reacts With Hydrochloric Acid.
From brainly.in
Balance equation for copper ll oxide reacts with dilute hydrochloric Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. this video demonstrates the action of acids on metal oxides. The substances used are copper oxide. Copper Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper Oxide Reacts With Hydrochloric Acid this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. Cuo + 2hcl → cucl2. cucl_2(aq) and the. Copper Oxide Reacts With Hydrochloric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide Reacts With Hydrochloric Acid The substances used are copper oxide and dilute. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. copper. Copper Oxide Reacts With Hydrochloric Acid.
From www.studocu.com
Copper chloride Chemistry lab Salt preparation. Reacting copper(II Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. cucl_2(aq) and. Copper Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
41 Cu + HCl Copper + Hydrochloric acid💚 YouTube Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. cuo + hcl. Copper Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Copper (II) oxide reacts with dilute hydrochloric acid. CuO(s Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. cucl_2(aq) and the reaction. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and. Copper Oxide Reacts With Hydrochloric Acid.
From www.tes.com
Metals and Acids Reactivity Series KS4 Edexcel 91 Teaching Resources Copper Oxide Reacts With Hydrochloric Acid copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii). Copper Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper Oxide Reacts With Hydrochloric Acid copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. The substances used are copper oxide and dilute. cucl_2(aq) and the reaction. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. this reaction is. Copper Oxide Reacts With Hydrochloric Acid.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper Oxide Reacts With Hydrochloric Acid cucl_2(aq) and the reaction. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. this video demonstrates the action of acids on metal oxides. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. The. Copper Oxide Reacts With Hydrochloric Acid.
From cedfqugf.blob.core.windows.net
Copper (I) Oxide And Hydrochloric Acid at Jeremy Dunn blog Copper Oxide Reacts With Hydrochloric Acid cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. copper oxide is basic, so it reacts with hydrochloric acid. Copper Oxide Reacts With Hydrochloric Acid.
From www.diomedia.com
STOCK IMAGE, , JF0694, 01B473E7 , Science Source Search Stock Photos Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. cuo + hcl. Copper Oxide Reacts With Hydrochloric Acid.
From www.sciencephoto.com
Copper in hydrochloric acid Stock Image A500/0755 Science Photo Copper Oxide Reacts With Hydrochloric Acid Cuo + 2hcl → cucl2. The substances used are copper oxide and dilute. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this video demonstrates the action of acids on metal oxides. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. this reaction is a type. Copper Oxide Reacts With Hydrochloric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide Reacts With Hydrochloric Acid Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo. Copper Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Hydrochloric Acid Reaction HCL and mixed metal copper oxides YouTube Copper Oxide Reacts With Hydrochloric Acid Cuo + 2hcl → cucl2. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. this video demonstrates the action of acids on metal oxides. copper oxide is basic, so it reacts with hydrochloric acid. Copper Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED How many grams of copper (I) chloride are formed if 5.000 g of Copper Oxide Reacts With Hydrochloric Acid cucl_2(aq) and the reaction. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo + 2hcl → cucl2. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii). Copper Oxide Reacts With Hydrochloric Acid.
From www.slideshare.net
Metals Reactivity Series Copper Oxide Reacts With Hydrochloric Acid The substances used are copper oxide and dilute. cucl_2(aq) and the reaction. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo + 2hcl → cucl2. cuo + hcl = cucl2 + h2o is. Copper Oxide Reacts With Hydrochloric Acid.
From brainly.in
What will be the color of solution when copper oxide and dilute Copper Oxide Reacts With Hydrochloric Acid copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. this video demonstrates the action of acids on metal oxides. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii). Copper Oxide Reacts With Hydrochloric Acid.
From www.sciencephoto.com
Copper (II) oxide reacts with hydrochloric acid Stock Image C036 Copper Oxide Reacts With Hydrochloric Acid The substances used are copper oxide and dilute. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. this reaction is a type of double replacement reaction called a neutralization. Copper Oxide Reacts With Hydrochloric Acid.
From cexoxhzj.blob.core.windows.net
Copper Oxide And Carbon Reaction at Tracey Miller blog Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. The substances used are copper oxide and dilute. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. Cuo + 2hcl → cucl2. this reaction is a type of double. Copper Oxide Reacts With Hydrochloric Acid.
From slidetodoc.com
SSLC ONLINE CLASSES Grade 10 Chapter 3 Subject Copper Oxide Reacts With Hydrochloric Acid Cuo + 2hcl → cucl2. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. The substances used are copper oxide and dilute. this video demonstrates the action of acids on metal oxides. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. cuo + hcl = cucl2. Copper Oxide Reacts With Hydrochloric Acid.
From www.gkseries.com
When copper oxide and dilute hydrochloric acid react, colour changes to Copper Oxide Reacts With Hydrochloric Acid Cuo + 2hcl → cucl2. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. cucl_2(aq) and the reaction. this reaction is a type of. Copper Oxide Reacts With Hydrochloric Acid.
From www.slideshare.net
Metals Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. this video demonstrates the action of acids on metal oxides. Cuo + 2hcl → cucl2. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. Copper Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Write equation for the following reactions (a) Zinc reacts Copper Oxide Reacts With Hydrochloric Acid copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. Cuo + 2hcl → cucl2. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this. Copper Oxide Reacts With Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Oxide Reacts With Hydrochloric Acid cucl_2(aq) and the reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. The substances used are copper oxide and dilute. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. this reaction is a type of. Copper Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Ag + HCl (Silver + Hydrochloric acid) Equation YouTube Copper Oxide Reacts With Hydrochloric Acid Cuo + 2hcl → cucl2. The substances used are copper oxide and dilute. this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and two. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl). Copper Oxide Reacts With Hydrochloric Acid.
From www.slideshare.net
Reaction types Copper Oxide Reacts With Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo + 2hcl → cucl2. cucl_2(aq) and the reaction. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl). Copper Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Reaction of Metallic oxides YouTube Copper Oxide Reacts With Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. this video demonstrates the action of acids on metal. Copper Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper Oxide Reacts With Hydrochloric Acid this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. Cuo + 2hcl → cucl2. copper oxide is basic, so it reacts with. Copper Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Copper & Hydrochloric Acid Ligand Substitution YouTube Copper Oxide Reacts With Hydrochloric Acid Cuo + 2hcl → cucl2. copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. The substances used are copper oxide and. Copper Oxide Reacts With Hydrochloric Acid.
From www.chegg.com
Solved F. Other Reactions 14. Copper(II)chloride and water Copper Oxide Reacts With Hydrochloric Acid The substances used are copper oxide and dilute. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. this video demonstrates the action of acids on metal oxides. cucl_2(aq) and the reaction. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l). Copper Oxide Reacts With Hydrochloric Acid.
From www.toppr.com
Take a small amount of copper oxide in a beaker and add dilute Copper Oxide Reacts With Hydrochloric Acid copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. cucl_2(aq) and the reaction. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. Cuo + 2hcl → cucl2. cuo + hcl = cucl2 +. Copper Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Copper oxide reacts with hydrochloric acid. Why is this an Copper Oxide Reacts With Hydrochloric Acid copper oxide is basic, so it reacts with hydrochloric acid to form copper chloride and water. Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. Cuo + 2hcl → cucl2. cucl_2(aq) and the reaction.. Copper Oxide Reacts With Hydrochloric Acid.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper Oxide Reacts With Hydrochloric Acid Cuo(s) + 2hcl(aq) rarr cucl_2(aq) + 2h_2o(l) in aqueous solution cu^(2+) exists as. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid copper(ii) oxide. cucl_2(aq) and the reaction. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2). Copper Oxide Reacts With Hydrochloric Acid.
From www.sciencephoto.com
Two copper oxide reactions Stock Image A500/0465 Science Photo Library Copper Oxide Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c l. this video demonstrates the action of acids on metal oxides. Cuo + 2hcl → cucl2. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. Copper Oxide Reacts With Hydrochloric Acid.