How Do You Protect Aluminum From Galvanic Corrosion . Less noble metal in this combination becomes the anode and corrodes. But how can we protect aluminum from all of these? corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. This blog will delve into aluminium protection and why it’s essential to guard against aluminium and stainless steels (austenitic, duplex and super duplex stainless steels) are sufficiently different. Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. Most designers know enough to be dangerous,. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. galvanic corrosion of aluminium. More noble metal becomes the cathode and is protected from corrosion by.
from blog.thepipingmart.com
galvanic corrosion of aluminium. But how can we protect aluminum from all of these? Less noble metal in this combination becomes the anode and corrodes. This blog will delve into aluminium protection and why it’s essential to guard against corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. Most designers know enough to be dangerous,. aluminium and stainless steels (austenitic, duplex and super duplex stainless steels) are sufficiently different. Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. More noble metal becomes the cathode and is protected from corrosion by.
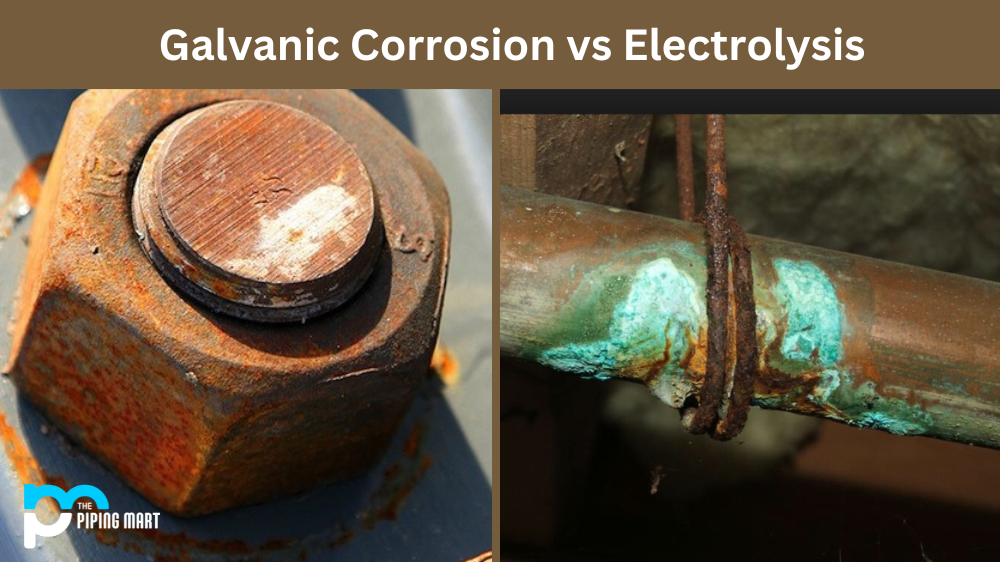
Galvanic Corrosion vs Electrolysis What's the Difference
How Do You Protect Aluminum From Galvanic Corrosion More noble metal becomes the cathode and is protected from corrosion by. More noble metal becomes the cathode and is protected from corrosion by. galvanic corrosion of aluminium. Most designers know enough to be dangerous,. Less noble metal in this combination becomes the anode and corrodes. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. aluminium and stainless steels (austenitic, duplex and super duplex stainless steels) are sufficiently different. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. But how can we protect aluminum from all of these? This blog will delve into aluminium protection and why it’s essential to guard against Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them.
From wilkinsoncoutts.com
Salt is bad for your health! Corrosion basics Wilkinson Coutts How Do You Protect Aluminum From Galvanic Corrosion More noble metal becomes the cathode and is protected from corrosion by. Most designers know enough to be dangerous,. Less noble metal in this combination becomes the anode and corrodes. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. But how can we protect aluminum from all of these? if you're an architect designing. How Do You Protect Aluminum From Galvanic Corrosion.
From www.eboss.co.nz
Galvanic Action Mismatching of Corrosion Protected Steel Products EBOSS How Do You Protect Aluminum From Galvanic Corrosion galvanic corrosion of aluminium. Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. Less noble metal in this combination becomes the anode and corrodes. aluminium and stainless steels (austenitic, duplex and super. How Do You Protect Aluminum From Galvanic Corrosion.
From suggesthow.com
How to Neutralize Aluminum Corrosion How Do You Protect Aluminum From Galvanic Corrosion Most designers know enough to be dangerous,. This blog will delve into aluminium protection and why it’s essential to guard against Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. More noble metal. How Do You Protect Aluminum From Galvanic Corrosion.
From monroeengineering.com
How to Protect Aluminum From Corrosion OneMonroe How Do You Protect Aluminum From Galvanic Corrosion Less noble metal in this combination becomes the anode and corrodes. This blog will delve into aluminium protection and why it’s essential to guard against furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. More noble metal becomes the cathode and is protected from corrosion by.. How Do You Protect Aluminum From Galvanic Corrosion.
From lovelolablog.com
How do you clean corrosion off of aluminum? Love Lola Blog How Do You Protect Aluminum From Galvanic Corrosion Most designers know enough to be dangerous,. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. But how can we protect aluminum from all of these? Less noble metal in this combination. How Do You Protect Aluminum From Galvanic Corrosion.
From gluethings.com
How can we prevent galvanic corrosion between aluminum and brass How Do You Protect Aluminum From Galvanic Corrosion Most designers know enough to be dangerous,. Less noble metal in this combination becomes the anode and corrodes. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. More noble metal becomes the cathode and is protected from corrosion by. galvanic corrosion of aluminium. corrosion. How Do You Protect Aluminum From Galvanic Corrosion.
From blog.thepipingmart.com
Galvanic Corrosion vs Electrolysis What's the Difference How Do You Protect Aluminum From Galvanic Corrosion if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. galvanic corrosion of aluminium. This blog will delve into aluminium protection and why it’s essential to guard against corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. Galvanic corrosion can occur when, when two different metals are in direct. How Do You Protect Aluminum From Galvanic Corrosion.
From mavink.com
Galvanic Corrosion Chart Dissimilar Metals How Do You Protect Aluminum From Galvanic Corrosion Most designers know enough to be dangerous,. More noble metal becomes the cathode and is protected from corrosion by. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. galvanic corrosion. How Do You Protect Aluminum From Galvanic Corrosion.
From www.researchgate.net
(PDF) A Review of Research on Galvanic Corrosion of Aluminum Alloys How Do You Protect Aluminum From Galvanic Corrosion furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. But how can we protect aluminum from all of these? if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. Less noble metal in this combination becomes the anode and corrodes. . How Do You Protect Aluminum From Galvanic Corrosion.
From www.wconline.com
What Galvanic Corrosion on Building Facades Can Lead To 20220311 How Do You Protect Aluminum From Galvanic Corrosion corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. galvanic corrosion of aluminium. But how can we protect aluminum from all of these? More noble metal becomes the cathode and is protected from. How Do You Protect Aluminum From Galvanic Corrosion.
From stevedmarineconsulting.com
Editorial Island Lady Fire Analyzed Feature Understanding and How Do You Protect Aluminum From Galvanic Corrosion Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. This blog will delve into aluminium protection and why it’s essential to guard against But how can we protect aluminum from all of these? corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. . How Do You Protect Aluminum From Galvanic Corrosion.
From blog.thepipingmart.com
How to Prevent Galvanic Corrosion Between Aluminum and Stainless Steel How Do You Protect Aluminum From Galvanic Corrosion More noble metal becomes the cathode and is protected from corrosion by. But how can we protect aluminum from all of these? furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion.. How Do You Protect Aluminum From Galvanic Corrosion.
From www.practicalmachinist.com
How to protect cast aluminum from corrosion? How Do You Protect Aluminum From Galvanic Corrosion if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. But how can we protect aluminum from all of these? corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. Less noble metal in this combination becomes the anode and corrodes. More noble metal becomes the cathode and is protected from. How Do You Protect Aluminum From Galvanic Corrosion.
From blog.thepipingmart.com
What is Galvanic Corrosion? How Do You Protect Aluminum From Galvanic Corrosion This blog will delve into aluminium protection and why it’s essential to guard against Most designers know enough to be dangerous,. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. More noble metal becomes the cathode and is protected from corrosion by. furthermore, it’s also possible to use the process of galvanic corrosion to. How Do You Protect Aluminum From Galvanic Corrosion.
From gluethings.com
How can we prevent galvanic corrosion between aluminum and brass How Do You Protect Aluminum From Galvanic Corrosion Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. galvanic corrosion of aluminium. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. But how can we protect aluminum from all of these? This blog will delve into aluminium protection and why. How Do You Protect Aluminum From Galvanic Corrosion.
From www.repairerdrivennews.com
Aluminum Association study of corrosion near hood hem offers lessons How Do You Protect Aluminum From Galvanic Corrosion Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. More. How Do You Protect Aluminum From Galvanic Corrosion.
From www.youtube.com
How To Protect Aluminum From Corrosion Vapor Honing Technologies How Do You Protect Aluminum From Galvanic Corrosion Less noble metal in this combination becomes the anode and corrodes. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. More noble metal becomes the cathode and is protected from corrosion by. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals.. How Do You Protect Aluminum From Galvanic Corrosion.
From mavink.com
Galvanic Corrosion Aluminum Stainless Steel How Do You Protect Aluminum From Galvanic Corrosion galvanic corrosion of aluminium. Less noble metal in this combination becomes the anode and corrodes. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. Most designers know enough to be dangerous,. More noble metal becomes the cathode and is protected from corrosion by. Galvanic corrosion can occur when, when two different metals are. How Do You Protect Aluminum From Galvanic Corrosion.
From blog.thepipingmart.com
How are Brass Objects Protected from Corrosion How Do You Protect Aluminum From Galvanic Corrosion furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. But how can we protect aluminum from all of these? Most designers know enough to be. How Do You Protect Aluminum From Galvanic Corrosion.
From www.youtube.com
Galvanic Corrosion what causes it and how to prevent, have an issue on How Do You Protect Aluminum From Galvanic Corrosion Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. Less noble metal in this combination becomes the anode and corrodes. More noble metal becomes the cathode and is protected from corrosion by. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by. How Do You Protect Aluminum From Galvanic Corrosion.
From exounhfkb.blob.core.windows.net
Stainless Steel And Aluminum Galvanic Corrosion at Hannah Sullivan blog How Do You Protect Aluminum From Galvanic Corrosion galvanic corrosion of aluminium. This blog will delve into aluminium protection and why it’s essential to guard against But how can we protect aluminum from all of these? More noble metal becomes the cathode and is protected from corrosion by. aluminium and stainless steels (austenitic, duplex and super duplex stainless steels) are sufficiently different. Most designers know enough. How Do You Protect Aluminum From Galvanic Corrosion.
From www.museoinclusivo.com
How to Neutralize Aluminum Corrosion Cleaning, Sealing, and More How Do You Protect Aluminum From Galvanic Corrosion This blog will delve into aluminium protection and why it’s essential to guard against Less noble metal in this combination becomes the anode and corrodes. More noble metal becomes the cathode and is protected from corrosion by. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. But how can we protect aluminum from all. How Do You Protect Aluminum From Galvanic Corrosion.
From highrise.nl
Galvanic corrosion in bolted Aluminium joints Highrise How Do You Protect Aluminum From Galvanic Corrosion But how can we protect aluminum from all of these? corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them.. How Do You Protect Aluminum From Galvanic Corrosion.
From blog.thepipingmart.com
Protecting Magnesium From Corrosion How Do You Protect Aluminum From Galvanic Corrosion if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. Most designers know enough to be dangerous,. Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. Less noble metal. How Do You Protect Aluminum From Galvanic Corrosion.
From orapiasia.com
Galvanic Corrosion An InDepth Analysis ORAPI Asia How Do You Protect Aluminum From Galvanic Corrosion Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. But how can we protect aluminum from all of these? furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. corrosion can appear in many. How Do You Protect Aluminum From Galvanic Corrosion.
From mungfali.com
Galvanic Corrosion Examples How Do You Protect Aluminum From Galvanic Corrosion This blog will delve into aluminium protection and why it’s essential to guard against Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. if you're an architect designing a metal exterior, you've probably. How Do You Protect Aluminum From Galvanic Corrosion.
From www.museoinclusivo.com
How to Prevent Galvanic Corrosion Between Aluminum and Stainless Steel How Do You Protect Aluminum From Galvanic Corrosion corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. Less noble metal in this combination becomes the anode and corrodes. galvanic corrosion of aluminium. Most designers know enough to be dangerous,. More noble metal becomes the cathode and is protected from corrosion by. furthermore, it’s also possible to use the process of galvanic. How Do You Protect Aluminum From Galvanic Corrosion.
From www.practicalmachinist.com
Cast aluminum/stainless bolt galvanic corrosion. Best chemical or How Do You Protect Aluminum From Galvanic Corrosion Galvanic corrosion can occur when, when two different metals are in direct contact and an electrolyte bridge is formed between them. Most designers know enough to be dangerous,. aluminium and stainless steels (austenitic, duplex and super duplex stainless steels) are sufficiently different. More noble metal becomes the cathode and is protected from corrosion by. This blog will delve into. How Do You Protect Aluminum From Galvanic Corrosion.
From strongtie.co.nz
Galvanic Action Mismatching of Corrosion Protected Steel Products How Do You Protect Aluminum From Galvanic Corrosion This blog will delve into aluminium protection and why it’s essential to guard against galvanic corrosion of aluminium. More noble metal becomes the cathode and is protected from corrosion by. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion.. How Do You Protect Aluminum From Galvanic Corrosion.
From www.balkanplumbing.com
Three Ways To Prevent Galvanic Corrosion In Your Pipes How Do You Protect Aluminum From Galvanic Corrosion galvanic corrosion of aluminium. But how can we protect aluminum from all of these? More noble metal becomes the cathode and is protected from corrosion by. Less noble metal in this combination becomes the anode and corrodes. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. furthermore, it’s also possible to use. How Do You Protect Aluminum From Galvanic Corrosion.
From www.neicorporation.com
New Selfhealing Anticorrosion Coating for ZincPlated & Galvanized How Do You Protect Aluminum From Galvanic Corrosion But how can we protect aluminum from all of these? corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. galvanic corrosion of aluminium. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. This blog will delve into aluminium protection and. How Do You Protect Aluminum From Galvanic Corrosion.
From metalprofy.com
How to Prevent Galvanic Corrosion Between Aluminum and Steel? MetalProfy How Do You Protect Aluminum From Galvanic Corrosion Less noble metal in this combination becomes the anode and corrodes. corrosion can appear in many forms, such as oxidation, pitting, or galvanic corrosion. if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. galvanic corrosion of aluminium. This blog will delve into aluminium protection and why it’s essential to guard against But. How Do You Protect Aluminum From Galvanic Corrosion.
From exonedycy.blob.core.windows.net
How To Fix Corrosion On Metal at Charles Sylvester blog How Do You Protect Aluminum From Galvanic Corrosion furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. But how can we protect aluminum from all of these? More noble metal becomes the cathode and is protected from corrosion by. Galvanic corrosion can occur when, when two different metals are in direct contact and an. How Do You Protect Aluminum From Galvanic Corrosion.
From blog.thepipingmart.com
Ability Of Aluminum Alloy To Resist Corrosion How Do You Protect Aluminum From Galvanic Corrosion This blog will delve into aluminium protection and why it’s essential to guard against if you're an architect designing a metal exterior, you've probably heard of galvanic corrosion. More noble metal becomes the cathode and is protected from corrosion by. furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to. How Do You Protect Aluminum From Galvanic Corrosion.
From chiangmaiplaces.net
How Is Galvanic Corrosion Prevented? The 11 Top Answers How Do You Protect Aluminum From Galvanic Corrosion furthermore, it’s also possible to use the process of galvanic corrosion to our advantage by using it to actually protect certain metals. Less noble metal in this combination becomes the anode and corrodes. More noble metal becomes the cathode and is protected from corrosion by. aluminium and stainless steels (austenitic, duplex and super duplex stainless steels) are sufficiently. How Do You Protect Aluminum From Galvanic Corrosion.