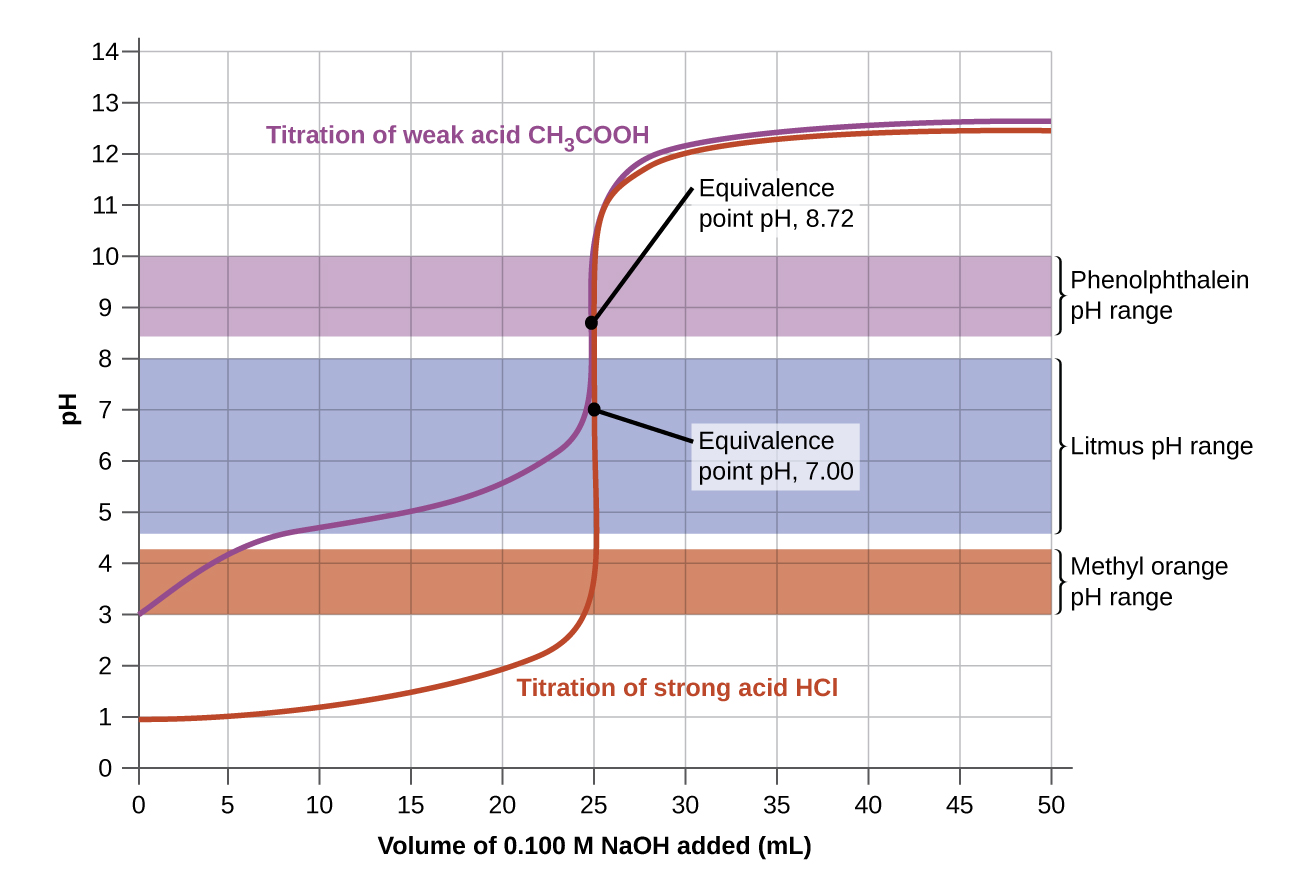
Ph Titration Results . To find the endpoint of a titration, you use an indicator. The equivalence point of a titration sorting out some confusing terms when. Naoh + hcl → nacl + h 2 o In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. An indicator changes colour when all of the unknown reactant is used up. Titrations of a strong acid with a strong base are the simplest to. Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results. The shape of the curve. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve.
from chem.libretexts.org
Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results. The equivalence point of a titration sorting out some confusing terms when. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. An indicator changes colour when all of the unknown reactant is used up. Titrations of a strong acid with a strong base are the simplest to. The shape of the curve. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. To find the endpoint of a titration, you use an indicator. Naoh + hcl → nacl + h 2 o In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a.
2.7 AcidBase Titrations Chemistry LibreTexts
Ph Titration Results An indicator changes colour when all of the unknown reactant is used up. The equivalence point of a titration sorting out some confusing terms when. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Titrations of a strong acid with a strong base are the simplest to. An indicator changes colour when all of the unknown reactant is used up. The shape of the curve. Naoh + hcl → nacl + h 2 o 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results. To find the endpoint of a titration, you use an indicator.
From www.youtube.com
Titration Lab Results and pH Chemistry Matters YouTube Ph Titration Results The shape of the curve. Titrations of a strong acid with a strong base are the simplest to. Naoh + hcl → nacl + h 2 o In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. The equivalence point of a titration sorting out. Ph Titration Results.
From www.researchgate.net
pH titration curves for different alkali metal systems on SDSTiP Ph Titration Results Naoh + hcl → nacl + h 2 o An indicator changes colour when all of the unknown reactant is used up. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. To find the endpoint of a titration, you use an indicator. In this section we. Ph Titration Results.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Ph Titration Results The equivalence point of a titration sorting out some confusing terms when. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Naoh + hcl → nacl + h 2 o To find the endpoint of a titration, you use an indicator. The shape of. Ph Titration Results.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Ph Titration Results An indicator changes colour when all of the unknown reactant is used up. Naoh + hcl → nacl + h 2 o Titrations of a strong acid with a strong base are the simplest to. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a.. Ph Titration Results.
From www.savemyexams.com
pH Titration Curves CIE A Level Chemistry Revision Notes 2022 Ph Titration Results In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. The equivalence point of a titration sorting out some confusing terms when. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid.. Ph Titration Results.
From www.researchgate.net
(PDF) Titration and pH Measurement Ph Titration Results Titrations of a strong acid with a strong base are the simplest to. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Careful. Ph Titration Results.
From www.slideserve.com
PPT Titration and pH Curves. PowerPoint Presentation, free download Ph Titration Results The equivalence point of a titration sorting out some confusing terms when. Titrations of a strong acid with a strong base are the simplest to. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0. Ph Titration Results.
From mmerevise.co.uk
pH Curves Questions and Revision MME Ph Titration Results The shape of the curve. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Naoh + hcl → nacl + h. Ph Titration Results.
From www.researchgate.net
pH titration curves of TRIS family (a) and morpholine family (b) at [T Ph Titration Results Naoh + hcl → nacl + h 2 o Titrations of a strong acid with a strong base are the simplest to. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The equivalence point of a titration sorting out some confusing terms when. The shape of the curve.. Ph Titration Results.
From byjus.com
The graph of pH during the titration of NaOH and HCl Ph Titration Results An indicator changes colour when all of the unknown reactant is used up. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. The shape of. Ph Titration Results.
From solvedlib.com
The following graph shows the pH curve for the titrat… SolvedLib Ph Titration Results In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. The shape of the curve. Naoh + hcl → nacl + h 2 o Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results.. Ph Titration Results.
From schoolworkhelper.net
pH Titration Lab Explained SchoolWorkHelper Ph Titration Results In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. To find the endpoint of a titration, you use an indicator. The shape of the curve. Plotting the ph of the solution in the flask against the amount of acid or base added produces a. Ph Titration Results.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Ph Titration Results Titrations of a strong acid with a strong base are the simplest to. Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. 50.0 cm 3 of 0.10. Ph Titration Results.
From saylordotorg.github.io
AcidBase Titrations Ph Titration Results Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The equivalence point of a titration sorting out some confusing terms when. Titrations of a strong acid with a strong base are the simplest to. The shape of the curve. 50.0 cm 3 of 0.10 mol dm 3 naoh. Ph Titration Results.
From chart-studio.plotly.com
Titration Curve(pH vs Volume of NaOH) line chart made by 7rat2 plotly Ph Titration Results Naoh + hcl → nacl + h 2 o Titrations of a strong acid with a strong base are the simplest to. To find the endpoint of a titration, you use an indicator. An indicator changes colour when all of the unknown reactant is used up. Plotting the ph of the solution in the flask against the amount of acid. Ph Titration Results.
From roqed.com
Titration (pH) curves ROQED Ph Titration Results The shape of the curve. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. The equivalence point of a titration sorting out some confusing terms when. An indicator changes colour when all of the unknown reactant is used up. Titrations of a strong acid with a. Ph Titration Results.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Ph Titration Results Titrations of a strong acid with a strong base are the simplest to. Naoh + hcl → nacl + h 2 o Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results. In this section we will learn how to calculate the ph of an analyte solution throughout the. Ph Titration Results.
From saylordotorg.github.io
AcidBase Titrations Ph Titration Results The equivalence point of a titration sorting out some confusing terms when. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The shape. Ph Titration Results.
From www.researchgate.net
Typical pHstat titration line of the PME assay (correlation Ph Titration Results The shape of the curve. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Naoh + hcl → nacl + h 2 o The equivalence point of a titration sorting out some confusing terms when. Titrations of a strong acid with a strong base are the simplest to.. Ph Titration Results.
From www.researchgate.net
Superposition of pH titration curves monitored by CD (j) and UV Ph Titration Results Naoh + hcl → nacl + h 2 o In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Titrations of a strong acid with a strong base are the simplest to. An indicator changes colour when all of the unknown reactant is used up.. Ph Titration Results.
From www.savemyexams.com
pH Titration Curves OCR A Level Chemistry Revision Notes 2017 Ph Titration Results Naoh + hcl → nacl + h 2 o 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. To find the endpoint of a titration, you use an indicator. Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential. Ph Titration Results.
From webmis.highland.cc.il.us
AcidBase Titrations Ph Titration Results In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. The shape of the curve. The equivalence point of a titration sorting. Ph Titration Results.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Ph Titration Results 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. An indicator changes colour when all of the unknown reactant is used up. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare. Ph Titration Results.
From www.researchgate.net
pH titration. (a) Time course of remaining sucrose Ph Titration Results The equivalence point of a titration sorting out some confusing terms when. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. To find the endpoint of a titration, you use an indicator. Naoh + hcl → nacl + h 2 o Careful selection of. Ph Titration Results.
From www.researchgate.net
pHtitration curves of polyotoluidine zirconium(IV)tungstate with Ph Titration Results To find the endpoint of a titration, you use an indicator. The shape of the curve. An indicator changes colour when all of the unknown reactant is used up. The equivalence point of a titration sorting out some confusing terms when. Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure. Ph Titration Results.
From chart-studio.plotly.com
pH Titration Phenol scatter chart made by Jacopodc plotly Ph Titration Results 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results. The shape of the curve. Naoh + hcl → nacl + h 2 o The equivalence. Ph Titration Results.
From www.chegg.com
This is a titration table and we have 50.0ml 0.1319M Ph Titration Results The equivalence point of a titration sorting out some confusing terms when. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid.. Ph Titration Results.
From www.savemyexams.com
pH Titration Curves CIE A Level Chemistry Revision Notes 2022 Ph Titration Results In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Titrations of a strong acid with a strong base are the simplest to. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The. Ph Titration Results.
From chem.libretexts.org
2.7 AcidBase Titrations Chemistry LibreTexts Ph Titration Results The equivalence point of a titration sorting out some confusing terms when. Titrations of a strong acid with a strong base are the simplest to. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. In this section we will learn how to calculate the ph of. Ph Titration Results.
From www.youtube.com
pH titration curve calculations for weak acid strong base YouTube Ph Titration Results Naoh + hcl → nacl + h 2 o To find the endpoint of a titration, you use an indicator. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. In this section we will learn how to calculate the ph of an analyte solution throughout the. Ph Titration Results.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Ph Titration Results The shape of the curve. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. Titrations of a strong acid with a. Ph Titration Results.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Ph Titration Results To find the endpoint of a titration, you use an indicator. The equivalence point of a titration sorting out some confusing terms when. Careful selection of indicators is necessary to obtain accurate results, and repetition of the titration is essential to ensure concordant results. In this section we will learn how to calculate the ph of an analyte solution throughout. Ph Titration Results.
From www.pearson.com
The following plot shows two pH titration curves, each representi Ph Titration Results The equivalence point of a titration sorting out some confusing terms when. The shape of the curve. Titrations of a strong acid with a strong base are the simplest to. Naoh + hcl → nacl + h 2 o An indicator changes colour when all of the unknown reactant is used up. Careful selection of indicators is necessary to obtain. Ph Titration Results.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Ph Titration Results In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. To find the endpoint of a titration, you use an indicator. Naoh + hcl → nacl + h 2 o An indicator changes colour when all of the unknown reactant is used up. Plotting the. Ph Titration Results.
From www.researchgate.net
Experimental pH titration curves of the protein termini and His. For Ph Titration Results 50.0 cm 3 of 0.10 mol dm 3 naoh is gradually added to 25.0 cm 3 of 0.15 mol dm 3 hydrochloric acid. Titrations of a strong acid with a strong base are the simplest to. The shape of the curve. To find the endpoint of a titration, you use an indicator. An indicator changes colour when all of the. Ph Titration Results.