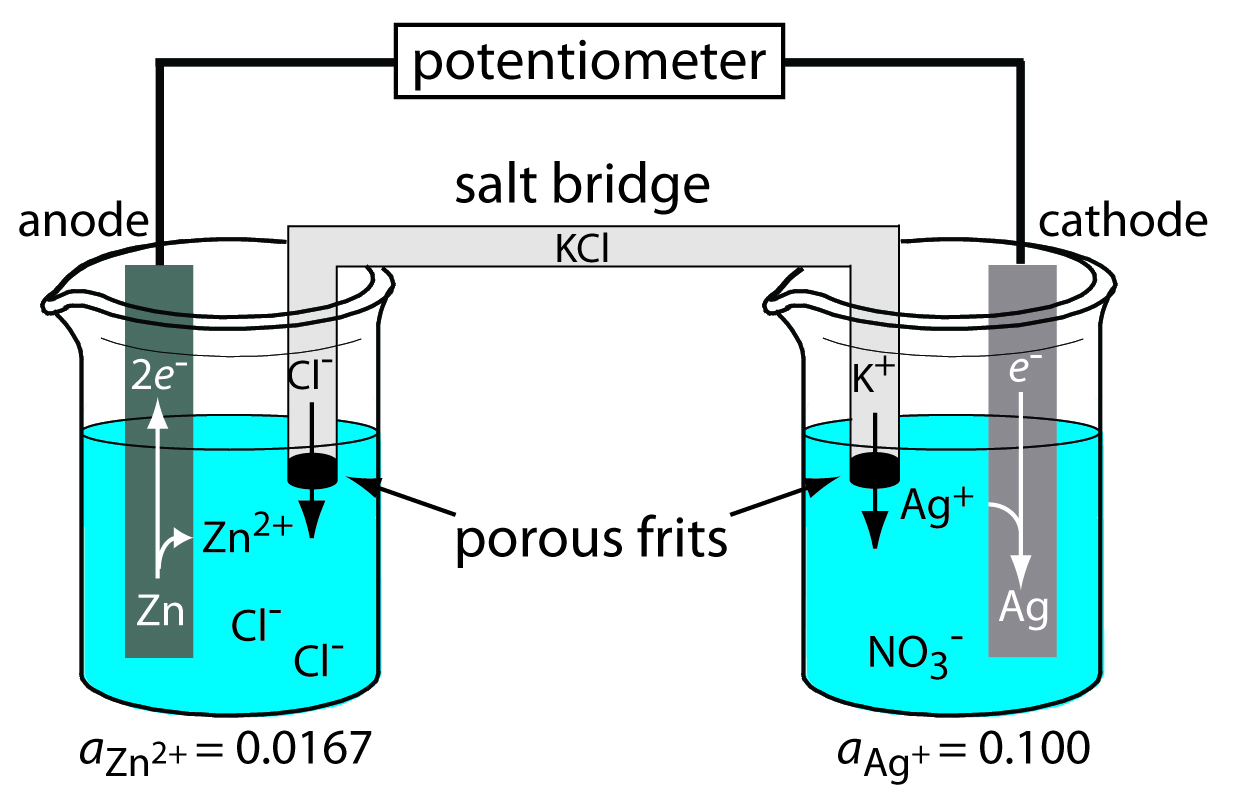
Zn Electrode Potential . An introduction to redox equilibria and electrode potentials. The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. This page explains the background to standard electrode potentials. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2.
from chem.libretexts.org
The convention is to put the more positive. 45 rows standard electrode potentials in aqueous solution at 25°c. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up.
11.2 Potentiometric Methods Chemistry LibreTexts
Zn Electrode Potential 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. This page explains the background to standard electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. An introduction to redox equilibria and electrode potentials. 45 rows standard electrode potentials in aqueous solution at 25°c. The convention is to put the more positive.
From questions-in.kunduz.com
The standard electrode potential of Zn, A... Physical Chemistry Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential. Zn Electrode Potential.
From slideplayer.com
ELECTROCHEMISTRY. ppt download Zn Electrode Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The convention is to put the more positive. This page explains the background to standard electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are. Zn Electrode Potential.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Zn Electrode Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Because the. Zn Electrode Potential.
From www.researchgate.net
(a) Single electrode potentials of a Zn anode in 6 M KOH and a Pb Zn Electrode Potential To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. This page explains the background to standard electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. Zn Electrode Potential.
From www.youtube.com
XI Chemistry Ch07 Lec02 (Determination of electrode potential of zinc Zn Electrode Potential 45 rows standard electrode potentials in aqueous solution at 25°c. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up.. Zn Electrode Potential.
From askfilo.com
Calculation of Standard Electrode potential Pt wire t gas zn Electrode 1 Zn Electrode Potential An introduction to redox equilibria and electrode potentials. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The convention is to put the more positive. This. Zn Electrode Potential.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the. Zn Electrode Potential.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2. This page explains the background to standard electrode potentials. An introduction to redox equilibria and electrode potentials. Because the zinc electrode in this. Zn Electrode Potential.
From pandai.me
Standard Electrode Potential Zn Electrode Potential The convention is to put the more positive. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up.. Zn Electrode Potential.
From questions-in.kunduz.com
The standard electrode potential of Zn, A... Physical Chemistry Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. 45 rows standard electrode potentials in aqueous solution at 25°c. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. This page explains the background to standard electrode. Zn Electrode Potential.
From www.slideserve.com
PPT Electrode Potentials PowerPoint Presentation, free download ID Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The convention is to put the more positive. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard electrode potential,. Zn Electrode Potential.
From www.numerade.com
SOLVEDkalculate the standard cell potential of the following cell at Zn Electrode Potential 45 rows standard electrode potentials in aqueous solution at 25°c. This page explains the background to standard electrode potentials. The convention is to put the more positive. An introduction to redox equilibria and electrode potentials. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. The standard electrode potential, eθ, of a half cell, zn2+(aq)/. Zn Electrode Potential.
From question.pandai.org
Standard Electrode Potential Zn Electrode Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The convention is to put the more positive. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. Electrode potentials can be used to calculate the. Zn Electrode Potential.
From slideplayer.com
ELECTROCHEMISTRY. ppt download Zn Electrode Potential 45 rows standard electrode potentials in aqueous solution at 25°c. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. An introduction to redox equilibria and electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq). Zn Electrode Potential.
From www.mdpi.com
Energies Free FullText Study on Electrode Potential of Zinc Nickel Zn Electrode Potential An introduction to redox equilibria and electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2. The convention is to put the more positive. Electrode potentials can be used to calculate. Zn Electrode Potential.
From www.alamy.com
Observed and calculated values for the standard electrode potentials Zn Electrode Potential This page explains the background to standard electrode potentials. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The convention. Zn Electrode Potential.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Zn Electrode Potential 45 rows standard electrode potentials in aqueous solution at 25°c. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Because the. Zn Electrode Potential.
From www.docbrown.info
Zinc Zn Chemistry Zn2+ compounds oxidation state +2 complexes complex Zn Electrode Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. 45. Zn Electrode Potential.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Zn Electrode Potential Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. An introduction to redox equilibria and electrode potentials. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. The convention is to put the more positive. Because the zinc electrode in this. Zn Electrode Potential.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Zn Electrode Potential The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The convention is to put the more positive. Electrode potentials can. Zn Electrode Potential.
From byjus.com
Variation of Cell Potential in ZnCu Cell Chemistry Practicals Class Zn Electrode Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. Because. Zn Electrode Potential.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Zn Electrode Potential To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. An introduction to redox equilibria and electrode potentials. The convention is to put the more positive. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are. Zn Electrode Potential.
From kunduz.com
[ANSWERED] The standard electrode potential of Zn Ag and Cu electrodes Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. This page explains the background to standard electrode potentials. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. 372 rows the data below tabulates standard electrode potentials. Zn Electrode Potential.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Zn Electrode Potential The convention is to put the more positive. An introduction to redox equilibria and electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the. Zn Electrode Potential.
From www.toppr.com
The standard electrode potentials for P^2 + Pb and Zn^2 + Zn are 0. Zn Electrode Potential 45 rows standard electrode potentials in aqueous solution at 25°c. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. Electrode potentials can be used to. Zn Electrode Potential.
From saylordotorg.github.io
Standard Potentials Zn Electrode Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to. Zn Electrode Potential.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Zn Electrode Potential An introduction to redox equilibria and electrode potentials. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The standard. Zn Electrode Potential.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5744606 Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). 45 rows standard electrode potentials in aqueous solution at 25°c. 372. Zn Electrode Potential.
From mungfali.com
Table Of Standard Electrode Potentials Zn Electrode Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. An introduction to redox equilibria and electrode potentials. Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while. Zn Electrode Potential.
From free-images.com
Free Images standard electrode potential zinc Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). Electrode potentials can be used to calculate the voltage produced when. Zn Electrode Potential.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the platinum surface, the standard electrode potential of the zn 2. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows. Zn Electrode Potential.
From chem.libretexts.org
6.2 Standard Electrode Potentials Chemistry LibreTexts Zn Electrode Potential The convention is to put the more positive. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. To determine the. Zn Electrode Potential.
From www.youtube.com
Determination of Standard Electrode potential of 'Zn and Cu Zn Electrode Potential Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at. 45 rows standard electrode potentials in aqueous solution at 25°c. The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up.. Zn Electrode Potential.
From slideplayer.com
Electrochemistry. ppt download Zn Electrode Potential An introduction to redox equilibria and electrode potentials. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following electrochemical cell. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). Because the zinc electrode in this cell. Zn Electrode Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Zn Electrode Potential 45 rows standard electrode potentials in aqueous solution at 25°c. The standard electrode potential, eθ, of a half cell, zn2+(aq)/ zn(s), is the emf represented by the cell diagram pt(s)|h2(g)|2h+(aq) ⁞⁞ zn2+(aq)|zn(s). Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. This page explains the background to standard electrode potentials. The convention is to. Zn Electrode Potential.