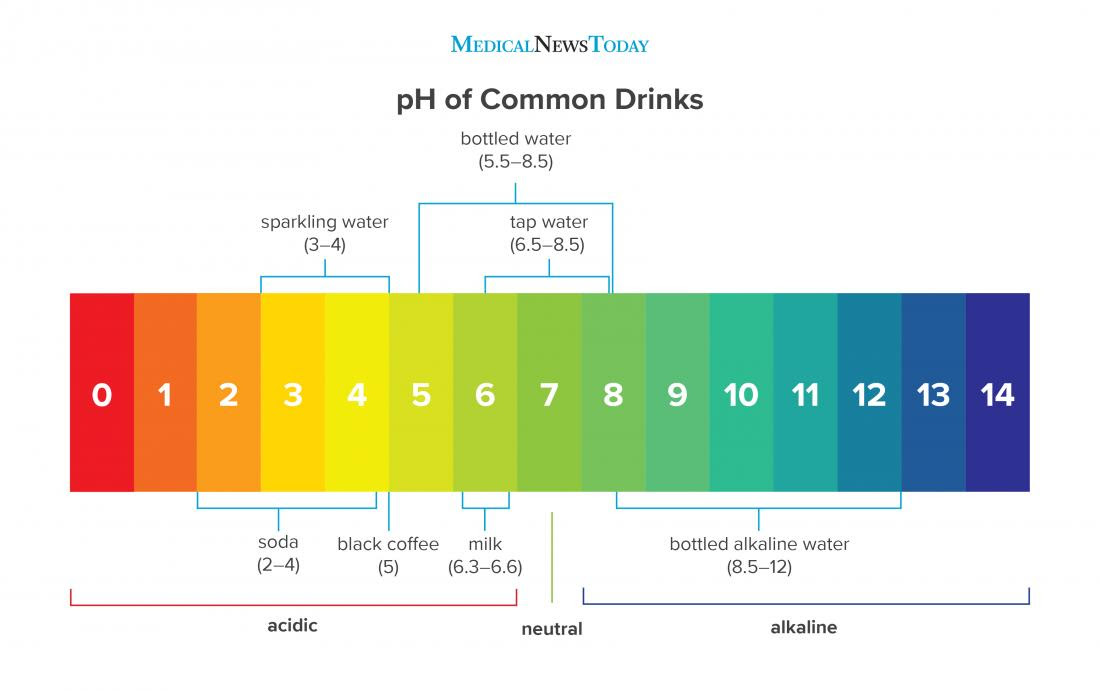
What Is The Ph Of Hot Water . Ph should be thought of simply as a number that indicates the basic or acidic. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. at room temperature (25 degrees celsius) the ph of pure water is 7. However, as the temperature increases,. If you increase the temperature to 100. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. ph generally from 0 (very acidic) to 14 (very alkaline). the ph of pure water is 7 at standard conditions (25°c and 1 atm).
from news.regenerativemedgroup.com
However, as the temperature increases,. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. the ph of pure water is 7 at standard conditions (25°c and 1 atm). Ph should be thought of simply as a number that indicates the basic or acidic. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. at room temperature (25 degrees celsius) the ph of pure water is 7. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. If you increase the temperature to 100.
The pH of water What to know Regenerative Medical Group
What Is The Ph Of Hot Water Ph should be thought of simply as a number that indicates the basic or acidic. However, as the temperature increases,. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. the ph of pure water is 7 at standard conditions (25°c and 1 atm). the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. at room temperature (25 degrees celsius) the ph of pure water is 7. Ph should be thought of simply as a number that indicates the basic or acidic. If you increase the temperature to 100.
From netsolwater.com
What is pH in water? How is pH level measured What Is The Ph Of Hot Water at room temperature (25 degrees celsius) the ph of pure water is 7. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is neutral on the. What Is The Ph Of Hot Water.
From mywaterearth.com
Safe pH Level for Drinking Water MyWaterEarth&Sky What Is The Ph Of Hot Water the ph of pure water is 7 at standard conditions (25°c and 1 atm). although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. at room temperature (25 degrees celsius) the ph of pure water is 7. the ph of water determines a substance’s solubility. What Is The Ph Of Hot Water.
From netsolwater.com
What are the pH and TDS in the water? How can it be controlled? Netsol What Is The Ph Of Hot Water Ph should be thought of simply as a number that indicates the basic or acidic. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. although the ph of pure water is 7, drinking water and natural water exhibits a. What Is The Ph Of Hot Water.
From skfelixer.com
The pH Value Of Purified Water — All You Need To Know SKF Elixer What Is The Ph Of Hot Water at room temperature (25 degrees celsius) the ph of pure water is 7. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. Ph should be thought of simply as a. What Is The Ph Of Hot Water.
From inspectapedia.com
WHO pH for drinking water, too high pH or too low pH acidic water What Is The Ph Of Hot Water at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. the ph of pure water is 7 at standard conditions (25°c and 1 atm). although the ph of pure. What Is The Ph Of Hot Water.
From www.water-rightgroup.com
Signs & Causes of Unbalanced Water pH Levels WaterRight What Is The Ph Of Hot Water However, as the temperature increases,. If you increase the temperature to 100. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. Ph should be thought of simply as a number. What Is The Ph Of Hot Water.
From www.survivopedia.com
How To Measure Water pH At Home Survivopedia What Is The Ph Of Hot Water If you increase the temperature to 100. Ph should be thought of simply as a number that indicates the basic or acidic. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. However, as the temperature increases,. ph generally from 0 (very acidic) to 14 (very alkaline). the ph of. What Is The Ph Of Hot Water.
From upberi.com
What to Know about the pH of Drinking Water (2022) What Is The Ph Of Hot Water at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. the ph of pure water is 7 at standard conditions (25°c and 1 atm). Ph should be thought of simply as a number that indicates the basic or acidic. ph generally from 0 (very acidic) to 14. What Is The Ph Of Hot Water.
From keywordsuggest.org
Image Gallery hydrochloric acid ph What Is The Ph Of Hot Water If you increase the temperature to 100. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. at room temperature (25 degrees celsius) the ph of pure water is 7. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. . What Is The Ph Of Hot Water.
From netsolwater.com
Why is pH important in measuring or determining water quality What Is The Ph Of Hot Water However, as the temperature increases,. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. the ph of water determines a substance’s solubility and biological availability of nutrients such as. What Is The Ph Of Hot Water.
From aquaclearws.com
Why Are The pH Levels Of Water So Important? What Is The Ph Of Hot Water the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. ph generally from 0 (very acidic) to 14 (very alkaline). the ph of pure water is. What Is The Ph Of Hot Water.
From techiescientist.com
Does Temperature Affect pH? Techiescientist What Is The Ph Of Hot Water However, as the temperature increases,. at room temperature (25 degrees celsius) the ph of pure water is 7. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is. What Is The Ph Of Hot Water.
From news.regenerativemedgroup.com
The pH of water What to know Regenerative Medical Group What Is The Ph Of Hot Water although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon.. What Is The Ph Of Hot Water.
From www.expii.com
What Is pH? — Definition & Overview Expii What Is The Ph Of Hot Water at room temperature (25 degrees celsius) the ph of pure water is 7. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of pure water is 7. What Is The Ph Of Hot Water.
From www.intec-america.com
Things You Must Know About pH Control and Drinking Water Treatment Intec America Corporation What Is The Ph Of Hot Water the ph of pure water is 7 at standard conditions (25°c and 1 atm). at room temperature (25 degrees celsius) the ph of pure water is 7. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. If you increase the temperature to 100. Ph should be thought of simply. What Is The Ph Of Hot Water.
From perfect-hydration.com
Drinking Water pH Explained What Should Be the Ideal pH? Perfect Hydration What Is The Ph Of Hot Water although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. at room temperature (25 degrees celsius) the ph of pure water is 7. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. If you increase the. What Is The Ph Of Hot Water.
From watertechadvice.com
pH of Water Everything You Need To Know What Is The Ph Of Hot Water ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Ph should be thought of simply as a number that indicates the basic or acidic. at 100°c, the ph of pure water is 6.14, which is considered. What Is The Ph Of Hot Water.
From zanyplantworld.com
PH Water Levels For The Plants Definition, Benefits, And Effects What Is The Ph Of Hot Water at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. the ph of pure water is 7 at standard conditions (25°c and 1 atm). Ph should. What Is The Ph Of Hot Water.
From www.worksheetsplanet.com
What is Ph Scale Definition of Ph Scale What Is The Ph Of Hot Water However, as the temperature increases,. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. the ph of pure water is 7 at standard conditions (25°c. What Is The Ph Of Hot Water.
From waterseer.org
What is pH in Water? Definition, Importance & Level Chart What Is The Ph Of Hot Water at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of pure water is 7 at standard conditions (25°c and 1 atm). Ph should be thought of simply as a number that indicates the basic or acidic. However, as the temperature increases,. If you increase the temperature to 100.. What Is The Ph Of Hot Water.
From waterseer.org
What is pH in Water? Definition, Importance & Level Chart What Is The Ph Of Hot Water at room temperature (25 degrees celsius) the ph of pure water is 7. If you increase the temperature to 100. the ph of pure water is 7 at standard conditions (25°c and 1 atm). the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. Ph should be thought. What Is The Ph Of Hot Water.
From blog.havells.com
Right pH Level in Drinking Water How Essential? Havells India Blog What Is The Ph Of Hot Water the ph of pure water is 7 at standard conditions (25°c and 1 atm). although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. If you increase the temperature to 100. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph. What Is The Ph Of Hot Water.
From chemguru.sg
How to Calculate pH of Water What Is The Ph Of Hot Water at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved.. What Is The Ph Of Hot Water.
From www.australianenvironmentaleducation.com.au
What is pH? pH is the abbreviation of the potential of Hydrogen What Is The Ph Of Hot Water If you increase the temperature to 100. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. However, as the temperature increases,. ph generally from 0 (very acidic) to 14 (very alkaline). Ph should be thought of simply as a number that indicates the basic or acidic.. What Is The Ph Of Hot Water.
From byjus.com
pH Of Acids And Bases Calculate pH Value Chemistry Byju's What Is The Ph Of Hot Water although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. However, as the temperature increases,. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. Ph should be thought of simply as a number that indicates the basic or acidic.. What Is The Ph Of Hot Water.
From mungfali.com
Water PH Level Chart What Is The Ph Of Hot Water Ph should be thought of simply as a number that indicates the basic or acidic. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. However, as the temperature increases,. . What Is The Ph Of Hot Water.
From waterseer.org
What is pH in Water? Definition, Importance & Level Chart What Is The Ph Of Hot Water at room temperature (25 degrees celsius) the ph of pure water is 7. the ph of pure water is 7 at standard conditions (25°c and 1 atm). at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. However, as the temperature increases,. Ph should be thought of. What Is The Ph Of Hot Water.
From dynamixinc.com
PH Alkalinity Mixing With Reaction Times Dynamix Agitators What Is The Ph Of Hot Water Ph should be thought of simply as a number that indicates the basic or acidic. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. at room temperature (25 degrees celsius) the ph of pure water is 7. ph generally from 0 (very acidic) to 14 (very. What Is The Ph Of Hot Water.
From purewaternutrition.com
Balancing PH with water and food What Is The Ph Of Hot Water at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Ph should be thought of simply as a number that. What Is The Ph Of Hot Water.
From www.mrunkplumbingheating.com
What Are PH Levels In Water And Why Do They Matter? Michael Runk Plumbing And Heating What Is The Ph Of Hot Water the ph of pure water is 7 at standard conditions (25°c and 1 atm). at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. the ph of water determines a substance’s solubility and biological availability of nutrients such as phosphorus, nitrogen, and carbon. If you increase the. What Is The Ph Of Hot Water.
From www.worksheetsplanet.com
What is PH Definition What Is The Ph Of Hot Water the ph of pure water is 7 at standard conditions (25°c and 1 atm). at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. If you increase the temperature to 100. However, as the temperature increases,. the ph of water determines a substance’s solubility and biological availability of nutrients such. What Is The Ph Of Hot Water.
From www.tardish2o.co.uk
PH water Levels pH level in Drinking Water What is a safe pH value? What Is The Ph Of Hot Water ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of pure water is 7 at standard conditions (25°c and 1 atm). although the ph of pure water is 7, drinking water and natural water exhibits a. What Is The Ph Of Hot Water.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog What Is The Ph Of Hot Water at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. the ph of pure water is 7 at standard conditions (25°c and 1 atm). However, as the temperature increases,. although the ph of pure water is 7, drinking water and natural water exhibits a ph range because. What Is The Ph Of Hot Water.
From destrixel.blogspot.com
Ph Level Of Water Alkaline Water 5 Popular Health Benefits of drinking it Environmental What Is The Ph Of Hot Water However, as the temperature increases,. at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of pure water is 7 at standard conditions (25°c and 1 atm). at room temperature (25 degrees celsius) the ph of pure water is 7. If you increase the temperature to 100. Ph. What Is The Ph Of Hot Water.
From www.plumbingsupply.com
Water pH and Your Plumbing What Is The Ph Of Hot Water although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved. ph generally from 0 (very acidic) to 14 (very alkaline). at 100°c, the ph of pure water is 6.14, which is considered neutral at this higher temperature. the ph of pure water is 7 at. What Is The Ph Of Hot Water.