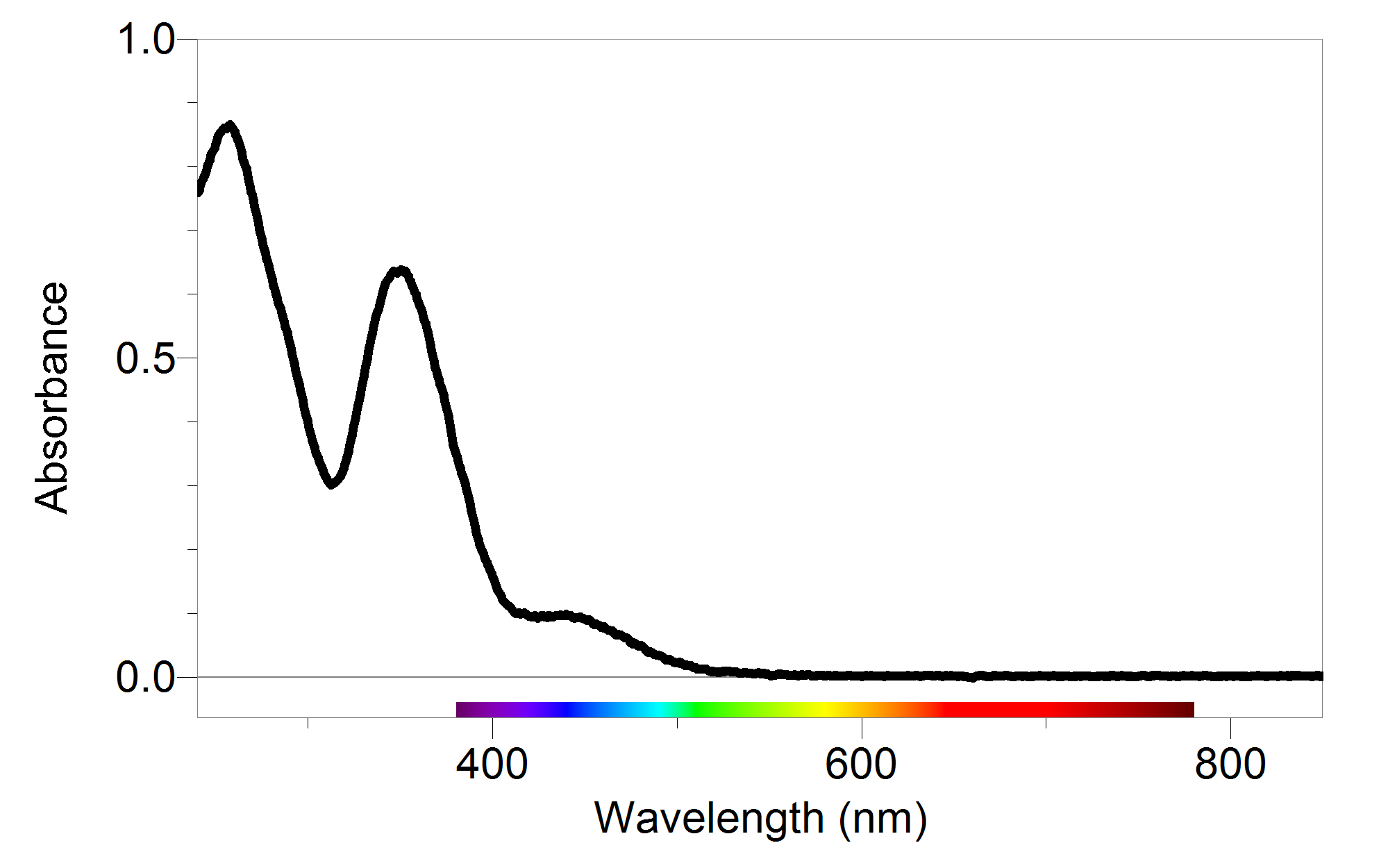
Standard Curve Vs Absorption Spectrum . Find out the definition, formula and. Find out how to calculate absorbance units and. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. A standard curve is a graph of light absorbance versus solution. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer.
from www.vernier.com
Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Find out the definition, formula and. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Find out how to calculate absorbance units and. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. A standard curve is a graph of light absorbance versus solution. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution.
Decoding Your Absorbance Readings Vernier
Standard Curve Vs Absorption Spectrum Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. A standard curve is a graph of light absorbance versus solution. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Find out how to calculate absorbance units and. Find out the definition, formula and.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Standard Curve Vs Absorption Spectrum A standard curve is a graph of light absorbance versus solution. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Find out the definition, formula and. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Find out how to. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Normalized absorption and fluorescence spectra of GFP and Cy3. The Standard Curve Vs Absorption Spectrum Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Atomic emission spectroscopy measures. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Absorption spectrum of PCA, wavelengths (nm) curve versus absorbance Standard Curve Vs Absorption Spectrum Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Find out how to calculate absorbance units and. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Absorbance spectra of phenol red at different pH values. Download Standard Curve Vs Absorption Spectrum Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Find out the definition, formula and. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. A standard curve is a graph of light absorbance versus solution. Find out. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Phenol red absorbance spectra after mixing 11 with a pHadjusted Standard Curve Vs Absorption Spectrum Find out the definition, formula and. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to measure the amount of light absorbed by a sample at a specific. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
2 Absorbance spectra solution of Riboflavin 5mM. Download Scientific Standard Curve Vs Absorption Spectrum Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Find out the. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
The spectral reflectance curve of vegetation. The major absorption and Standard Curve Vs Absorption Spectrum A standard curve is a graph of light absorbance versus solution. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Find out how to calculate absorbance units and. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Atomic. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Spectra of absorption (curve 1) and luminescence (curve 2, k exc ¼ 530 Standard Curve Vs Absorption Spectrum Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Atomic emission. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
UVVis absorption spectra of EGCG in the presence (a) and absence (b Standard Curve Vs Absorption Spectrum Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Find. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Ofloxacin UV absorption spectrum and standard curve. Download Standard Curve Vs Absorption Spectrum Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. A standard curve. Standard Curve Vs Absorption Spectrum.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Standard Curve Vs Absorption Spectrum Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Find out the. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Normalized absorbance and emission intensity spectra versus wavelength Standard Curve Vs Absorption Spectrum Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. A. Standard Curve Vs Absorption Spectrum.
From rheolution.com
Understanding Absorbance at Specific Wavelengths Standard Curve Vs Absorption Spectrum Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Find out the definition, formula and. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted. Standard Curve Vs Absorption Spectrum.
From cscdb.nku.edu
Spectrophotometry & Dilutions Standard Curve Vs Absorption Spectrum Find out how to calculate absorbance units and. A standard curve is a graph of light absorbance versus solution. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Atomic emission spectroscopy measures the intensity. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
An absorption spectrum for β carotene standard ( a ). An absorption Standard Curve Vs Absorption Spectrum Find out the definition, formula and. Find out how to calculate absorbance units and. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. A standard curve is a graph of light absorbance versus solution. Learn how to use a standard curve to determine the concentration of a solution. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Absorption spectra recorded for solutions after 3h reduction of 1mM NAD Standard Curve Vs Absorption Spectrum Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Find out the definition, formula and. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Find out how to calculate absorbance units and. Atomic emission spectroscopy measures the intensity of light emitted. Standard Curve Vs Absorption Spectrum.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Standard Curve Vs Absorption Spectrum Find out how to calculate absorbance units and. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. A standard curve is a graph of light absorbance versus solution. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Learn how to prepare. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
UVVis absorbance spectrum of melanin for 0.1, 0.2, 0.4, 0.6, and Standard Curve Vs Absorption Spectrum Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Find out how to. Standard Curve Vs Absorption Spectrum.
From quizlet.com
Absorption Spectrum lab Diagram Quizlet Standard Curve Vs Absorption Spectrum Find out how to calculate absorbance units and. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to use a standard curve to determine the concentration of a solution. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Standard absorption curve of MB solution. Download Scientific Diagram Standard Curve Vs Absorption Spectrum Find out the definition, formula and. A standard curve is a graph of light absorbance versus solution. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Find out how to calculate. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
UVVis absorption spectra of PNP at different pH values and Standard Curve Vs Absorption Spectrum Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Find out how to calculate absorbance units and. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Learn how to use a standard curve to determine the concentration of a. Standard Curve Vs Absorption Spectrum.
From www.masterorganicchemistry.com
Interpreting IR Specta A Quick Guide Master Organic Chemistry Standard Curve Vs Absorption Spectrum Find out the definition, formula and. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Find out how to calculate absorbance units and. Learn how to use a standard curve to determine. Standard Curve Vs Absorption Spectrum.
From www.vernier.com
Decoding Your Absorbance Readings Vernier Standard Curve Vs Absorption Spectrum Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer.. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
UVvisible absorption spectrum of AuNPs. A UVvisible absorption Standard Curve Vs Absorption Spectrum Find out the definition, formula and. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
UV absorption spectra of (A) TMZ and (B) IR820. Standard curves of (C Standard Curve Vs Absorption Spectrum Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Find out how to calculate absorbance units and. Find out the definition, formula and. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use a spectrophotometer to measure the. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Absorption spectrum of ICG with concentrations at 25 , 2.5 , 250 nM and Standard Curve Vs Absorption Spectrum Find out the definition, formula and. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. A standard curve is a graph of light absorbance versus solution. Atomic emission spectroscopy measures the intensity. Standard Curve Vs Absorption Spectrum.
From www.semanticscholar.org
Figure 3 from Ultraviolet absorption spectrum of chlorine peroxide Standard Curve Vs Absorption Spectrum Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. A. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
The spectral reflectance curve of vegetation. The major absorption and Standard Curve Vs Absorption Spectrum Find out the definition, formula and. A standard curve is a graph of light absorbance versus solution. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Find out how. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
The standard curve of (IV) concentration vs. absorbance. Download Standard Curve Vs Absorption Spectrum Learn how to use a spectrophotometer to measure the amount of light absorbed or transmitted by molecules in a solution. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Find. Standard Curve Vs Absorption Spectrum.
From websites.umich.edu
Chem 125 Experiment II Standard Curve Vs Absorption Spectrum Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. A standard curve is a graph of light absorbance versus solution. Find out how to calculate absorbance units and. Atomic emission spectroscopy. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Calibration curve of absorbance versus concentration. Download Standard Curve Vs Absorption Spectrum Find out how to calculate absorbance units and. Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Find out the definition, formula and. Learn how to prepare solutions, perform serial dilutions, and use a. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
Normalized excitation (or absorbance) and emission spectra of FPs of Standard Curve Vs Absorption Spectrum Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to measure the amount of light absorbed by a sample at a specific wavelength using a spectrometer. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed.. Standard Curve Vs Absorption Spectrum.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Standard Curve Vs Absorption Spectrum Learn how to use a standard curve to determine the concentration of a solution from absorbance data. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Find out how to calculate absorbance units and. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure. Standard Curve Vs Absorption Spectrum.
From courses.lumenlearning.com
Vision Anatomy and Physiology I Standard Curve Vs Absorption Spectrum Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to prepare solutions, perform serial dilutions, and use a spectrophotometer to measure absorbance and generate a standard curve. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. A standard. Standard Curve Vs Absorption Spectrum.
From www.researchgate.net
TROLOX CONCENTRATION VS ABSORBANCE FOR ABTS STANDARD CURVE. Download Standard Curve Vs Absorption Spectrum Learn how to use beer's law to measure the concentration of a species in a sample using spectroscopy. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Learn how to use a standard curve to determine the concentration of a solution from absorbance data. A standard curve is. Standard Curve Vs Absorption Spectrum.