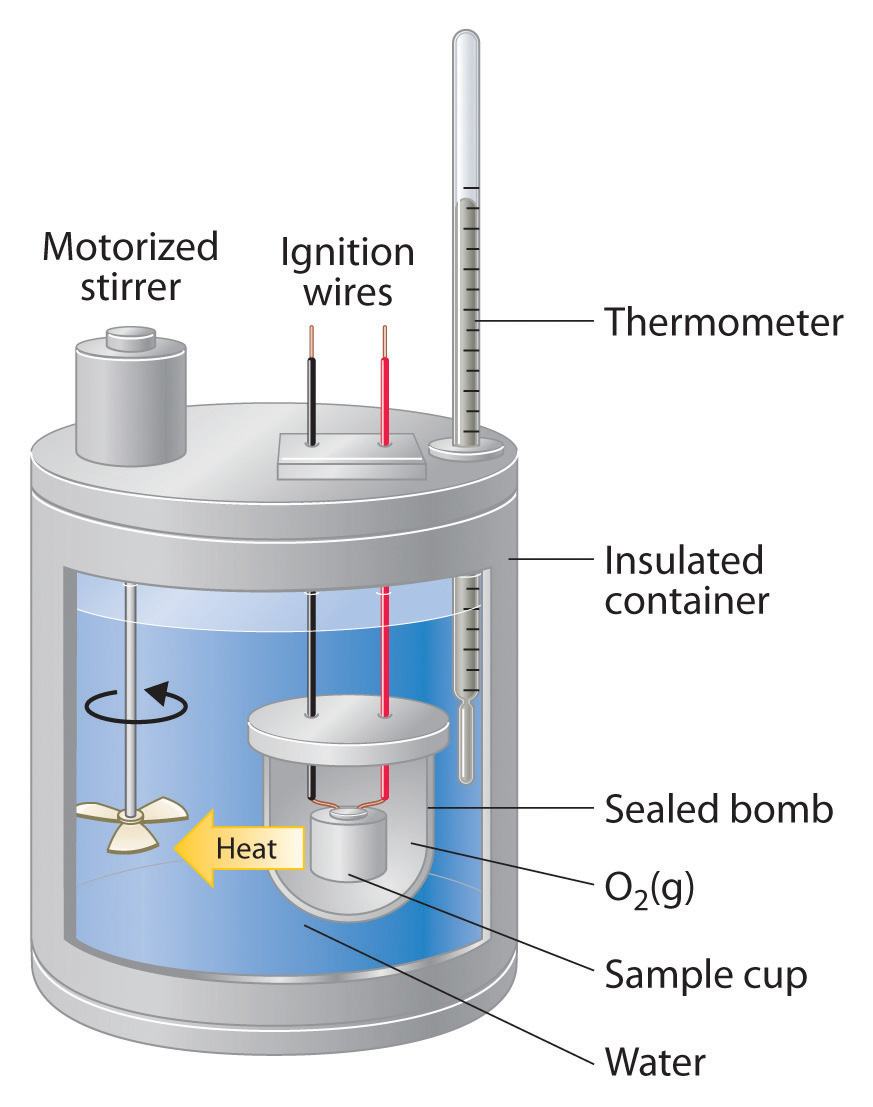
Write Principle Of Calorimetry Class 11 . A system is defined as an isolated one if no exchange or transfer of heat occurs between the. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. the principle of calorimetry. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. See diagrams, formulas and solved examples of. Find the formula, components, and.
from saylordotorg.github.io
learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. the principle of calorimetry. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. Find the formula, components, and. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. thermochemistry is the study of heat and energy changes in physical and chemical transformations.
Calorimetry
Write Principle Of Calorimetry Class 11 See examples of calorimeter problems and solutions, and test your knowledge with a quiz. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. Find the formula, components, and. See diagrams, formulas and solved examples of. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. the principle of calorimetry.
From www.youtube.com
CaLoRiMeTrY Principle of Calorimetry Method Of Mixtures Class 10 Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. the principle of calorimetry. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. . Write Principle Of Calorimetry Class 11.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Write Principle Of Calorimetry Class 11 Find the formula, components, and. the principle of calorimetry. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Thermodynamics 05 Bomb Calorimeter Class 11/JEE RAFTAAR YouTube Write Principle Of Calorimetry Class 11 See examples of calorimeter problems and solutions, and test your knowledge with a quiz. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. See diagrams, formulas and solved examples. Write Principle Of Calorimetry Class 11.
From www.studypool.com
SOLUTION Calorimetry class 11th Studypool Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. learn. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Class 11 chemistry Thermodynamics (calorific value of fuel and Bomb Write Principle Of Calorimetry Class 11 See examples of calorimeter problems and solutions, and test your knowledge with a quiz. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. Find the formula, components, and. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. A system is defined. Write Principle Of Calorimetry Class 11.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Write Principle Of Calorimetry Class 11 learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. the principle of calorimetry. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. Find the formula, components, and. See examples of calorimeter problems and solutions, and test. Write Principle Of Calorimetry Class 11.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Write Principle Of Calorimetry Class 11 learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. the principle of calorimetry. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. A system is defined as an isolated one if no exchange or transfer of heat occurs between the.. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Calorimetry calculation YouTube Write Principle Of Calorimetry Class 11 See diagrams, formulas and solved examples of. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of. Write Principle Of Calorimetry Class 11.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Write Principle Of Calorimetry Class 11 See examples of calorimeter problems and solutions, and test your knowledge with a quiz. the principle of calorimetry. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the. Write Principle Of Calorimetry Class 11.
From www.thoughtco.com
Calorimeter Definition in Chemistry Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. See diagrams, formulas and solved examples of. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn how to measure the heat transfer between two bodies using calorimetry,. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Glass Calorimeter 11th class chemistry ch.no.7 YouTube Write Principle Of Calorimetry Class 11 Find the formula, components, and. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. See diagrams, formulas and solved examples of. the principle of calorimetry. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. learn. Write Principle Of Calorimetry Class 11.
From physicspracticalreadings.blogspot.com
Class 11 Physics practical reading To measure internal diameter and Write Principle Of Calorimetry Class 11 Find the formula, components, and. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. See diagrams, formulas and. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Bomb Calorimeter Class 11th Thermodynamics Physics wallah Write Principle Of Calorimetry Class 11 the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of. Write Principle Of Calorimetry Class 11.
From saylordotorg.github.io
Calorimetry Write Principle Of Calorimetry Class 11 Find the formula, components, and. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. See diagrams, formulas and solved examples of. thermochemistry is the study of heat and energy changes in physical and chemical transformations.. Write Principle Of Calorimetry Class 11.
From dxoinieui.blob.core.windows.net
Calorimeter Diagram Class 11 at Ollie Stringer blog Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. See diagrams, formulas and solved examples of. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of. Write Principle Of Calorimetry Class 11.
From www.studypool.com
SOLUTION Calorimetry class 11th Studypool Write Principle Of Calorimetry Class 11 thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. Find the formula, components, and. See diagrams, formulas and solved examples of. learn about the measurement of heat energy using calorimeters, thermometers and specific heat.. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Class 11th Chemistry Calorimetry Problem 5.6 Chapter 5 Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. Find the formula, components, and. . Write Principle Of Calorimetry Class 11.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. Find the formula, components, and. See. Write Principle Of Calorimetry Class 11.
From www.studypool.com
SOLUTION Physics Calorimetry Class Notes Studypool Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. Find the formula, components, and. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn how to measure the heat transfer between. Write Principle Of Calorimetry Class 11.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Write Principle Of Calorimetry Class 11 learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. Find the formula, components, and. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. the principle of calorimetry. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures.. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Write Principle Of Calorimetry Class 11 thermochemistry is the study of heat and energy changes in physical and chemical transformations. See diagrams, formulas and solved examples of. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. learn how to measure the heat absorbed or released in a chemical or physical. Write Principle Of Calorimetry Class 11.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Write Principle Of Calorimetry Class 11 learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. See examples of calorimeter problems. Write Principle Of Calorimetry Class 11.
From studylib.net
Chapter 5b (PowerPoint) Write Principle Of Calorimetry Class 11 Find the formula, components, and. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. See diagrams, formulas and solved examples of. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. thermochemistry is the study of heat and energy changes in. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Calorimetry Principle of Calorimetry Class 11NEET IIT Physics Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. A. Write Principle Of Calorimetry Class 11.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Write Principle Of Calorimetry Class 11 learn about the measurement of heat energy using calorimeters, thermometers and specific heat. thermochemistry is the study of heat and energy changes in physical and chemical transformations. the principle of calorimetry. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. the principle of calorimetry states that heat lost by a body. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Principle of calorimetry class 11 NEET & JEE Physics class 11,unit Write Principle Of Calorimetry Class 11 the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. Find the formula, components, and. learn how to measure the heat absorbed or released in a chemical or physical process using a. Write Principle Of Calorimetry Class 11.
From users.highland.edu
Calorimetry Write Principle Of Calorimetry Class 11 A system is defined as an isolated one if no exchange or transfer of heat occurs between the. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter.. Write Principle Of Calorimetry Class 11.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Write Principle Of Calorimetry Class 11 thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. Find the formula,. Write Principle Of Calorimetry Class 11.
From dxoinieui.blob.core.windows.net
Calorimeter Diagram Class 11 at Ollie Stringer blog Write Principle Of Calorimetry Class 11 thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. See examples of calorimeter problems and solutions, and. Write Principle Of Calorimetry Class 11.
From www.studypool.com
SOLUTION Calorimetry class 11th Studypool Write Principle Of Calorimetry Class 11 Find the formula, components, and. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. See diagrams, formulas and solved examples of. learn. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Class 11th Physics Specific Heat Capacity Calorimetry Example 10. Write Principle Of Calorimetry Class 11 learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. See diagrams, formulas and solved examples of. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. Find the formula, components,. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Class 11 Physics Calorimetry 16 Principle Of Calorimetry For Write Principle Of Calorimetry Class 11 A system is defined as an isolated one if no exchange or transfer of heat occurs between the. the principle of calorimetry states that heat lost by a body at higher temperature equals heat gained by a body at lower. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter.. Write Principle Of Calorimetry Class 11.
From dxoinieui.blob.core.windows.net
Calorimeter Diagram Class 11 at Ollie Stringer blog Write Principle Of Calorimetry Class 11 learn about the measurement of heat energy using calorimeters, thermometers and specific heat. learn the fundamental principle of calorimetry and how to use a calorimeter to measure high temperatures. learn how to measure the heat absorbed or released in a chemical or physical process using a calorimeter. thermochemistry is the study of heat and energy changes. Write Principle Of Calorimetry Class 11.
From www.youtube.com
Principle of Calorimeter [ Law of Mixture ] Class 11 Physics (Hindi Write Principle Of Calorimetry Class 11 learn how to measure the heat transfer between two bodies using calorimetry, a method based on the law of conservation of energy. See examples of calorimeter problems and solutions, and test your knowledge with a quiz. thermochemistry is the study of heat and energy changes in physical and chemical transformations. Find the formula, components, and. learn about. Write Principle Of Calorimetry Class 11.
From www.scribd.com
Calorimetry Class 11 PDF Write Principle Of Calorimetry Class 11 thermochemistry is the study of heat and energy changes in physical and chemical transformations. learn about the measurement of heat energy using calorimeters, thermometers and specific heat. See diagrams, formulas and solved examples of. A system is defined as an isolated one if no exchange or transfer of heat occurs between the. learn the fundamental principle of. Write Principle Of Calorimetry Class 11.