Calorimetry Experiment Analysis . You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. The change in temperature of. Calorimetry is the measurement enthalpy changes in chemical reactions. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. There are two types of calorimetry experiments you need to know:.
from www.nagwa.com
The change in temperature of. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. There are two types of calorimetry experiments you need to know:. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Calorimetry is the measurement enthalpy changes in chemical reactions.
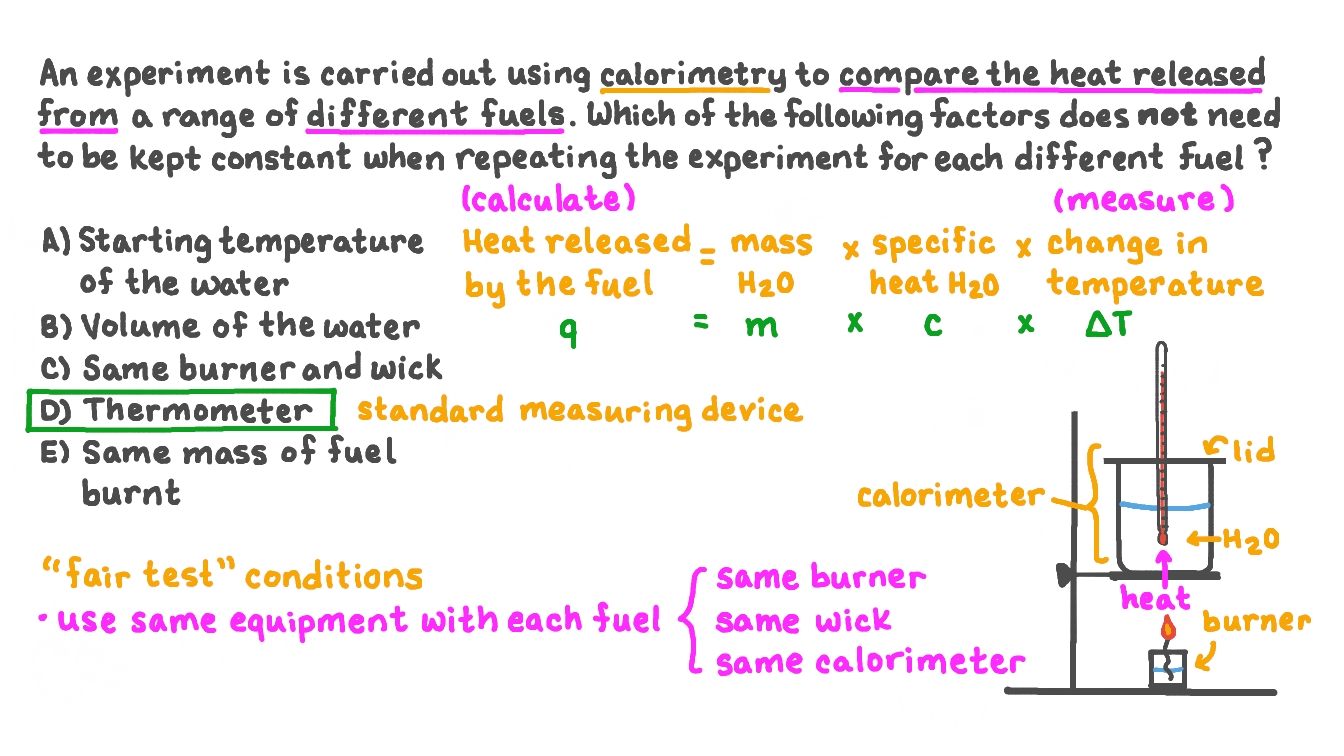
Question Video Identifying the Factor That Does Not Need to Be Held
Calorimetry Experiment Analysis In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. The change in temperature of. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. Calorimetry is the measurement enthalpy changes in chemical reactions. There are two types of calorimetry experiments you need to know:. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Calorimetry Experiment Analysis Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. There are two types of calorimetry experiments you need to know:. Calorimetry is a technique used to measure changes in. Calorimetry Experiment Analysis.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Experiment Analysis You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. There are two types of calorimetry experiments you need to know:. The change in temperature of. Calorimetry is the measurement enthalpy changes in chemical reactions.. Calorimetry Experiment Analysis.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Experiment Analysis You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. There are two types of calorimetry experiments you need to know:.. Calorimetry Experiment Analysis.
From webapi.bu.edu
⚡ The cold equations analysis. The Cold Equation By Tom Godwin Analysis Calorimetry Experiment Analysis Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is the measurement enthalpy changes in chemical reactions. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. You will perform several different calorimetry experiments and use the temperature. Calorimetry Experiment Analysis.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Experiment Analysis Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the measurement enthalpy changes in chemical reactions. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is. Calorimetry Experiment Analysis.
From www.creative-biostructure.com
Isothermal Titration Calorimetry (ITC) for Coronavirus Research Calorimetry Experiment Analysis Calorimetry is used to measure amounts of heat transferred to or from a substance. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. Calorimetry is the measurement enthalpy changes in chemical reactions. There are two types of calorimetry experiments you need to know:. To do so, the heat is exchanged with a calibrated object (calorimeter). In. Calorimetry Experiment Analysis.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Experiment Analysis The change in temperature of. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Hcl (aq) + naoh (aq) →. Calorimetry Experiment Analysis.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Experiment Analysis In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal,. Calorimetry Experiment Analysis.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Experiment Analysis In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Calorimetry is a technique used to measure. Calorimetry Experiment Analysis.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog Calorimetry Experiment Analysis Calorimetry is the measurement enthalpy changes in chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. To do so, the heat is exchanged with a calibrated object (calorimeter).. Calorimetry Experiment Analysis.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimetry Experiment Analysis Calorimetry is the measurement enthalpy changes in chemical reactions. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Calorimetry is used to measure amounts of heat transferred to or from a substance. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l). Calorimetry Experiment Analysis.
From klaiijxiw.blob.core.windows.net
Bomb Calorimeter Graph at Jennie Gaskins blog Calorimetry Experiment Analysis Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). There are two types of calorimetry experiments you need to know:. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. For example, if a dilute solution of a strong acid. Calorimetry Experiment Analysis.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Experiment Analysis Calorimetry is the measurement enthalpy changes in chemical reactions. There are two types of calorimetry experiments you need to know:. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. You will perform several different calorimetry experiments and use the temperature changes to. Calorimetry Experiment Analysis.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Experiment Analysis Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. There are two types of calorimetry experiments you need to know:. Calorimetry. Calorimetry Experiment Analysis.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Experiment Analysis Calorimetry is used to measure amounts of heat transferred to or from a substance. The change in temperature of. To do so, the heat is exchanged with a calibrated object (calorimeter). Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. Calorimetry is the measurement enthalpy changes in chemical reactions. In this experiment you will heat a. Calorimetry Experiment Analysis.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Analysis For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. Calorimetry is the measurement enthalpy changes in chemical reactions. In this experiment you will heat a known mass of a metal. Calorimetry Experiment Analysis.
From www.degruyter.com
Thermal Analysis and Calorimetry Calorimetry Experiment Analysis There are two types of calorimetry experiments you need to know:. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter. Calorimetry Experiment Analysis.
From www.pinterest.com
Pin on Botany Lessons Calorimetry Experiment Analysis To do so, the heat is exchanged with a calibrated object (calorimeter). You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. Calorimetry Experiment Analysis.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Experiment Analysis Calorimetry is the measurement enthalpy changes in chemical reactions. To do so, the heat is exchanged with a calibrated object (calorimeter). You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. There are two types. Calorimetry Experiment Analysis.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams Calorimetry Experiment Analysis Calorimetry is used to measure amounts of heat transferred to or from a substance. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a. Calorimetry Experiment Analysis.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Experiment Analysis In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is used to measure amounts of heat transferred to or from a substance. The change in temperature of. You will perform several different calorimetry experiments and use the temperature changes to calculate. Calorimetry Experiment Analysis.
From exoyqaivb.blob.core.windows.net
Bomb Calorimeter Class 11 at Nicholas Josey blog Calorimetry Experiment Analysis For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. To do so, the heat is exchanged with a calibrated object (calorimeter). In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Calorimetry Experiment Analysis.
From saylordotorg.github.io
Calorimetry Calorimetry Experiment Analysis Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. There are two types of calorimetry experiments you need to know:. Calorimetry is the measurement enthalpy changes in chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. The change in temperature of. You will perform several different calorimetry experiments and use. Calorimetry Experiment Analysis.
From www.baamboozle.com
Heat Gain and Heat Loss Baamboozle Baamboozle The Most Fun Calorimetry Experiment Analysis The change in temperature of. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do. Calorimetry Experiment Analysis.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Experiment Analysis To do so, the heat is exchanged with a calibrated object (calorimeter). There are two types of calorimetry experiments you need to know:. Calorimetry is the measurement enthalpy changes in chemical reactions. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Calorimetry is. Calorimetry Experiment Analysis.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held Calorimetry Experiment Analysis Calorimetry is the measurement enthalpy changes in chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. There are two types of calorimetry experiments you need to know:. Calorimetry is used to measure amounts of heat transferred to or from a substance. You will perform several different calorimetry experiments and use the temperature changes to. Calorimetry Experiment Analysis.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Experiment Analysis To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. For example, if a dilute solution of a strong acid (hcl) is reacted. Calorimetry Experiment Analysis.
From users.highland.edu
Calorimetry Calorimetry Experiment Analysis Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the measurement enthalpy changes in chemical. Calorimetry Experiment Analysis.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Experiment Analysis The change in temperature of. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the measurement enthalpy changes in chemical reactions. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. There are two types of calorimetry experiments. Calorimetry Experiment Analysis.
From joiwmxbys.blob.core.windows.net
Calorimetry With Two Solutions at Angelica Kirkland blog Calorimetry Experiment Analysis Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. There are two types of calorimetry experiments. Calorimetry Experiment Analysis.
From sites.google.com
3.2.1 (e) Q=mcΔT Ellesmere OCR A level Chemistry Calorimetry Experiment Analysis The change in temperature of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. There are two types of calorimetry experiments you need to know:. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution. Calorimetry Experiment Analysis.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Experiment Analysis Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. There are two types of calorimetry experiments you need to know:. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. The change in temperature of. To do so, the heat. Calorimetry Experiment Analysis.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Experiment Analysis To do so, the heat is exchanged with a calibrated object (calorimeter). You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. Calorimetry is the measurement enthalpy changes in chemical reactions. Calorimetry is used to. Calorimetry Experiment Analysis.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Experiment Analysis The change in temperature of. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong. Calorimetry Experiment Analysis.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Experiment Analysis For example, if a dilute solution of a strong acid (hcl) is reacted with a dilute solution of a strong base (naoh), the reaction is written. Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat. To do so, the heat is exchanged with a calibrated object (calorimeter). There are two types of calorimetry experiments you need. Calorimetry Experiment Analysis.