Clinical Trials Edc Systems . electronic data capture systems have become a very popular tool to use in managing data in clinical trials. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. Learn about the key features of. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. discover the role of edc systems in clinical research. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings.
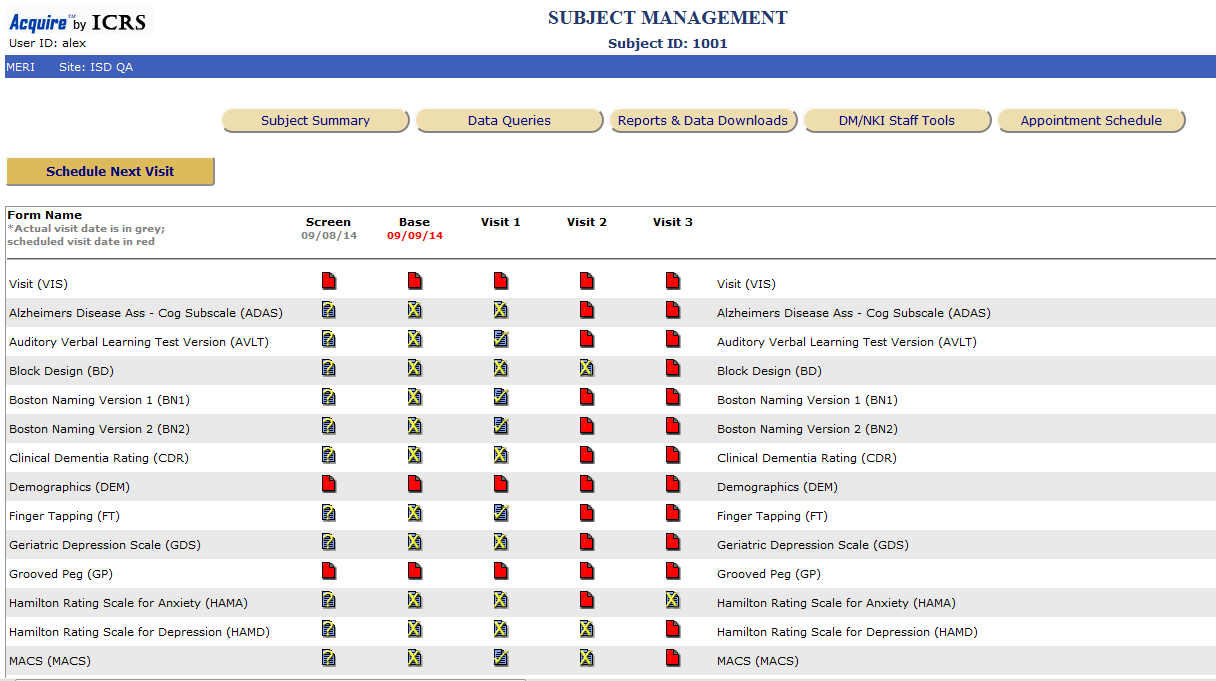
from icrs.rfmh.org
electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. edc in clinical trials is a software system for collecting, managing, and storing data. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. discover the role of edc systems in clinical research. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. Learn about the key features of. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture systems have become a very popular tool to use in managing data in clinical trials.
Electronic Data Capture EDC ACASI Clinical Research Software
Clinical Trials Edc Systems electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. electronic data capture systems have become a very popular tool to use in managing data in clinical trials. Learn about the key features of. edc in clinical trials is a software system for collecting, managing, and storing data. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. discover the role of edc systems in clinical research.
From www.postingstation.com
EDC Clinical Trials (Electronic Data Capture) Posting Station Clinical Trials Edc Systems electronic data capture systems have become a very popular tool to use in managing data in clinical trials. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. Learn about the key features of. . Clinical Trials Edc Systems.
From www.castoredc.com
Electronic Data Capture (EDC) Platform for Clinical Trials Clinical Trials Edc Systems electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. An edc system can help increase efficiency, ensure data. Clinical Trials Edc Systems.
From www.clinvigilant.com
Embracing Innovation The Future of Clinical Trials with Electronic Clinical Trials Edc Systems electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. explore the benefits and challenges of implementing edc. Clinical Trials Edc Systems.
From clinicalpursuit.com
Benefits of Electronic Data Capture Systems Clinical Trials Edc Systems electronic data capture systems have become a very popular tool to use in managing data in clinical trials. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. Learn about. Clinical Trials Edc Systems.
From clinicalpursuit.com
EDC systems for Clinical Trials Have they kept up with new technologies Clinical Trials Edc Systems discover the role of edc systems in clinical research. Learn about the key features of. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. electronic data capture systems have become a very popular tool to use in managing data in clinical trials. an electronic data capture (edc) system for. Clinical Trials Edc Systems.
From pepgra.com
Uses and Implementation process of Electronic Data Capture (EDC) in Clinical Trials Edc Systems electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. an electronic data capture (edc) system. Clinical Trials Edc Systems.
From milo-healthcare.com
Significance & Services of EDC Systems in Clinical Trials Clinical Trials Edc Systems An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. an electronic data capture (edc). Clinical Trials Edc Systems.
From www.mosio.com
What is an EDC in Clinical Trials Key Features and Benefits Mosio Clinical Trials Edc Systems electronic data capture systems have become a very popular tool to use in managing data in clinical trials. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. edc in clinical trials is a software system for collecting, managing, and storing data. explore the. Clinical Trials Edc Systems.
From www.mosio.com
What is an EDC in Clinical Trials Key Features and Benefits Mosio Clinical Trials Edc Systems An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. discover the role of edc systems in clinical research. Learn about the key features of. Learn how electronic data. Clinical Trials Edc Systems.
From www.altexsoft.com
Clinical Trial Software EDC, CTMS, ePRO, RTSM AltexSoft Clinical Trials Edc Systems discover the role of edc systems in clinical research. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. Learn about the key features of. edc in clinical trials is a software system for collecting, managing, and storing data. Learn how electronic data capture streamlines data management for efficient and accurate. Clinical Trials Edc Systems.
From clinicalpursuit.com
EDC systems for Clinical Trials Have they kept up with new technologies Clinical Trials Edc Systems an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. Learn how electronic data capture streamlines data management for. Clinical Trials Edc Systems.
From riseapps.co
EDC Systems in Clinical Trials Developers Guide Riseapps Clinical Trials Edc Systems electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. Learn about the key features of. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help. Clinical Trials Edc Systems.
From www.altexsoft.com
Clinical Trial Software EDC, CTMS, ePRO, RTSM AltexSoft Clinical Trials Edc Systems Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture in clinical trial (edc) has transformed the way clinical trials. Clinical Trials Edc Systems.
From clinicalpursuit.com
Benefits of Clinical EDC Data Management Software Clinical Trials Edc Systems Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. discover the role of edc systems in clinical research. edc in clinical trials is a software system for collecting, managing, and. Clinical Trials Edc Systems.
From credevo.com
Electronic Data Capture (EDC) In Clinical Trials Credevo Articles Clinical Trials Edc Systems an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. electronic data capture (edc) has become an integral. Clinical Trials Edc Systems.
From riseapps.co
EDC Systems in Clinical Trials Developers Guide Riseapps Clinical Trials Edc Systems An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. edc in clinical trials is a software system for collecting, managing, and storing data. Learn how electronic data capture. Clinical Trials Edc Systems.
From clinicalpursuit.com
Digital Clinical Trials EDC Systems ClinicalPURSUIT Clinical Trials Edc Systems explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. electronic data capture systems have become a very popular tool to use in managing data in clinical trials. Learn about the key features of. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials,. Clinical Trials Edc Systems.
From icrs.rfmh.org
Electronic Data Capture EDC ACASI Clinical Research Software Clinical Trials Edc Systems An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. edc in clinical trials is a software system for collecting, managing, and storing data. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. explore the benefits and challenges of implementing. Clinical Trials Edc Systems.
From riseapps.co
EDC Systems in Clinical Trials Developers Guide Riseapps Clinical Trials Edc Systems edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store,. Clinical Trials Edc Systems.
From www.altexsoft.com
Clinical Trial Software EDC, CTMS, ePRO, RTSM AltexSoft Clinical Trials Edc Systems an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Learn about the key features of. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. Learn how electronic data. Clinical Trials Edc Systems.
From www.linkedin.com
Why Clinical EDC Systems Are Crucial to the Success of Clinical Trials Clinical Trials Edc Systems electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture systems have become a very. Clinical Trials Edc Systems.
From within3.com
EDC Clinical Trials Within3 Clinical Trials Edc Systems electronic data capture systems have become a very popular tool to use in managing data in clinical trials. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. Learn about the key features of. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. an electronic data. Clinical Trials Edc Systems.
From clinnovo.blogspot.com
Clinnovo News Electronic Data Capture (EDC) During Clinical Trials Clinical Trials Edc Systems electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or. Clinical Trials Edc Systems.
From www.medidata.com
Addressing the Complexity of Phase 1 Clinical Trials with EDC Systems Clinical Trials Edc Systems discover the role of edc systems in clinical research. edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture systems have become a very popular tool to use in managing data in clinical trials. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted.. Clinical Trials Edc Systems.
From clinicalpursuit.com
Benefits of Clinical EDC Software Infographic Clinical Trials Edc Systems explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. an electronic data capture (edc) system for clinical trials or studies is. Clinical Trials Edc Systems.
From www.clinicaltrialsarena.com
How should EDC systems evolve to better serve modern clinical trials Clinical Trials Edc Systems explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. Learn about the key features of. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. discover the role of edc. Clinical Trials Edc Systems.
From medelink.ca
DataCapt EDC Clinical Trial Electronic Data Capture Software Clinical Trials Edc Systems An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier. Clinical Trials Edc Systems.
From www.altexsoft.com
Clinical Trial Software EDC, CTMS, ePRO, RTSM Clinical Trials Edc Systems an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. Learn how electronic data capture streamlines data management for efficient. Clinical Trials Edc Systems.
From riseapps.co
EDC Systems in Clinical Trials Developers Guide Riseapps Clinical Trials Edc Systems electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. electronic data capture (edc) has become an integral part of running clinical trials efficiently and effectively. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect,. Clinical Trials Edc Systems.
From www.clinicalleader.com
Has The Evolution Of EDC Kept Up With The Changes In Clinical Trials Clinical Trials Edc Systems an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. discover the role of edc systems in clinical research. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted.. Clinical Trials Edc Systems.
From riseapps.co
EDC Systems in Clinical Trials Developers Guide Riseapps Clinical Trials Edc Systems electronic data capture systems have become a very popular tool to use in managing data in clinical trials. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. edc. Clinical Trials Edc Systems.
From www.linkedin.com
A Brief Look at Electronic Data Capture (EDC) in Clinical Trials Clinical Trials Edc Systems An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. edc in clinical trials is a software system for collecting, managing, and storing data. explore the benefits and challenges of implementing. Clinical Trials Edc Systems.
From www.castoredc.com
Electronic Data Capture (EDC) in Clinical Trials Essential Guide Clinical Trials Edc Systems an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. Learn about the. Clinical Trials Edc Systems.
From zlynger.com
An Introduction to EDC Systems in Clinical Trials Zlynger Clinical Trials Edc Systems Learn about the key features of. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. Learn how electronic data capture streamlines data management for efficient and accurate clinical trials. electronic data capture in clinical trial (edc) has transformed the way clinical trials are conducted. discover the role of edc systems. Clinical Trials Edc Systems.
From www.socra.org
The Future of Clinical Trials Using Electronic Data Capture Systems SoCRA Clinical Trials Edc Systems edc in clinical trials is a software system for collecting, managing, and storing data. explore the benefits and challenges of implementing edc in clinical trials, research studies, and healthcare settings. An edc system can help increase efficiency, ensure data quality, reduce the time and cost of clinical trials, and help meet regulatory compliance. an electronic data capture. Clinical Trials Edc Systems.