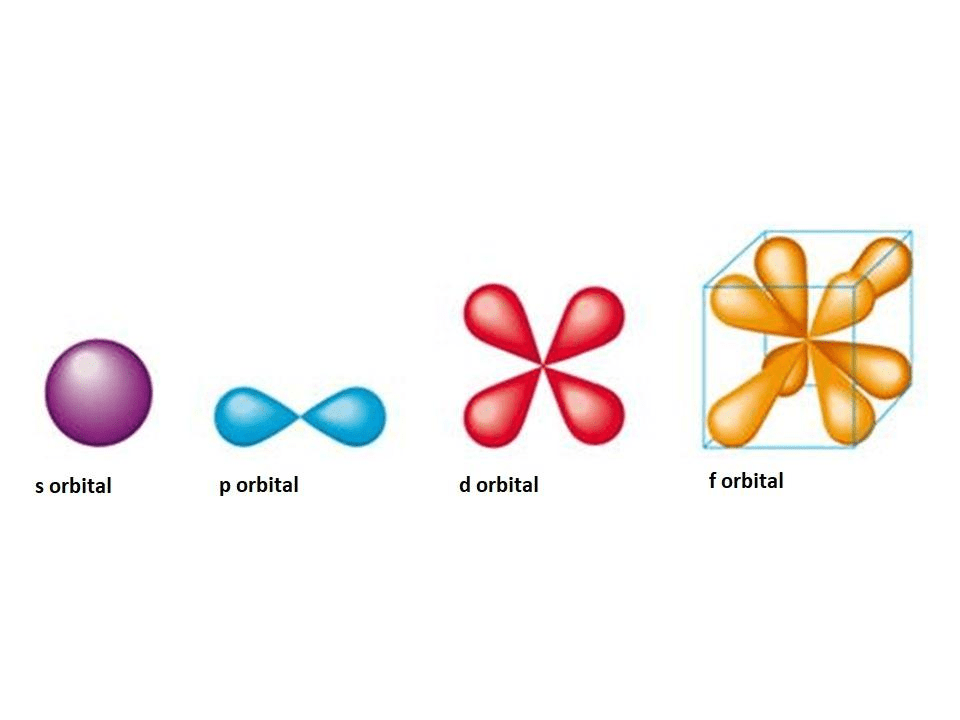
What Is An Orbital Shell In Chemistry . Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. An atomic orbital is characterized by three quantum numbers. The first character indicates the shell (n = 2 or n = 4). The principal quantum number, n, can be any positive integer. Each atomic orbital can be occupied by a maximum of two electrons. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. The general region for value of energy of the orbital and the average.
from www.vedantu.com
Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The first character indicates the shell (n = 2 or n = 4). The principal quantum number, n, can be any positive integer. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. An atomic orbital is characterized by three quantum numbers. The general region for value of energy of the orbital and the average. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Each atomic orbital can be occupied by a maximum of two electrons.
Define an atomic orbital.
What Is An Orbital Shell In Chemistry An atomic orbital is characterized by three quantum numbers. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. The first character indicates the shell (n = 2 or n = 4). The general region for value of energy of the orbital and the average. An atomic orbital is characterized by three quantum numbers. Each atomic orbital can be occupied by a maximum of two electrons. The principal quantum number, n, can be any positive integer. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica What Is An Orbital Shell In Chemistry An atomic orbital is characterized by three quantum numbers. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The first character indicates the shell (n = 2 or n =. What Is An Orbital Shell In Chemistry.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts What Is An Orbital Shell In Chemistry The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The general region for value of energy of the orbital and the average. The principal quantum number, n,. What Is An Orbital Shell In Chemistry.
From www.youtube.com
ORBIT vs ORBITAL electrons 3min Quick short differences YouTube What Is An Orbital Shell In Chemistry The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the. What Is An Orbital Shell In Chemistry.
From www.slideserve.com
PPT Shells and Subshells PowerPoint Presentation, free download ID What Is An Orbital Shell In Chemistry Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The general region for value of energy of the orbital and the average. Each atomic orbital can be occupied by a maximum of two electrons. The orbitals in an atom are organized into different layers around the nucleus called electron. What Is An Orbital Shell In Chemistry.
From www.youtube.com
What are shells,subshells and orbitals? Difference between shells What Is An Orbital Shell In Chemistry The principal quantum number, n, can be any positive integer. Each atomic orbital can be occupied by a maximum of two electrons. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. The general region for value of energy of the orbital and the average. Orbitals exist at specific energy levels. What Is An Orbital Shell In Chemistry.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is An Orbital Shell In Chemistry The general region for value of energy of the orbital and the average. An atomic orbital is characterized by three quantum numbers. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The first character indicates the shell (n = 2 or n = 4). The electrons. What Is An Orbital Shell In Chemistry.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is An Orbital Shell In Chemistry The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The principal quantum number, n, can be any positive integer. An atomic orbital is characterized by three quantum numbers. The first character indicates the shell (n = 2 or n = 4). The orbitals in an atom. What Is An Orbital Shell In Chemistry.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is An Orbital Shell In Chemistry The general region for value of energy of the orbital and the average. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The electrons in an atom. What Is An Orbital Shell In Chemistry.
From www.youtube.com
mr i explains Electron Shells (GCSE Chemistry) YouTube What Is An Orbital Shell In Chemistry The general region for value of energy of the orbital and the average. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. An atomic orbital is characterized by three quantum numbers. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between. What Is An Orbital Shell In Chemistry.
From www.britannica.com
Shell atomic model Description, Configuration, Chemistry, Proposed By What Is An Orbital Shell In Chemistry The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. Each atomic orbital can be occupied by a maximum of two electrons. An atomic orbital is characterized by three quantum numbers. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from. What Is An Orbital Shell In Chemistry.
From www.animalia-life.club
Electron Shell Diagram What Is An Orbital Shell In Chemistry The general region for value of energy of the orbital and the average. The first character indicates the shell (n = 2 or n = 4). Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The orbitals in an atom are organized into different layers around the nucleus called. What Is An Orbital Shell In Chemistry.
From byjus.com
Orbitals Chemistry (Shapes of Atomic Orbitals) Shape of s, p, d, and What Is An Orbital Shell In Chemistry The first character indicates the shell (n = 2 or n = 4). The principal quantum number, n, can be any positive integer. An atomic orbital is characterized by three quantum numbers. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Each shell is subdivided into subshells,. What Is An Orbital Shell In Chemistry.
From www.vedantu.com
Define an atomic orbital. What Is An Orbital Shell In Chemistry Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The first character indicates the shell (n = 2 or n = 4). Orbitals exist at specific energy. What Is An Orbital Shell In Chemistry.
From byjus.com
what is the sequence of shell , subshell, orbitals , orbits , electrons What Is An Orbital Shell In Chemistry The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in. What Is An Orbital Shell In Chemistry.
From www.animalia-life.club
Electron Shell Diagram What Is An Orbital Shell In Chemistry Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The principal quantum number, n, can be any positive integer. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Each atomic orbital can be occupied by a. What Is An Orbital Shell In Chemistry.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is An Orbital Shell In Chemistry An atomic orbital is characterized by three quantum numbers. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The first character indicates the shell (n = 2 or n = 4). Each shell is subdivided into subshells, which are made up of orbitals, each of which. What Is An Orbital Shell In Chemistry.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Is An Orbital Shell In Chemistry The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. Each shell is subdivided into subshells, which are made up of orbitals, each of which. What Is An Orbital Shell In Chemistry.
From socratic.org
What is the difference between electron shells and electron orbitals What Is An Orbital Shell In Chemistry The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The general region for value of energy of the orbital and the average. The first character indicates the. What Is An Orbital Shell In Chemistry.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is An Orbital Shell In Chemistry The first character indicates the shell (n = 2 or n = 4). The general region for value of energy of the orbital and the average. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The orbitals in an atom are organized into different layers around the nucleus called. What Is An Orbital Shell In Chemistry.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube What Is An Orbital Shell In Chemistry The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The general region for value of energy of the orbital and the average. Each atomic orbital can be occupied by a maximum of two electrons. The first character indicates the shell (n = 2 or n = 4).. What Is An Orbital Shell In Chemistry.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 What Is An Orbital Shell In Chemistry The principal quantum number, n, can be any positive integer. An atomic orbital is characterized by three quantum numbers. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the. What Is An Orbital Shell In Chemistry.
From courses.lumenlearning.com
Atoms and Elements Biology for Majors I What Is An Orbital Shell In Chemistry Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. The first character indicates the shell (n = 2 or n = 4). The principal quantum number, n, can be any. What Is An Orbital Shell In Chemistry.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is An Orbital Shell In Chemistry An atomic orbital is characterized by three quantum numbers. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The general region for value of energy of the orbital and the average. The first character indicates the shell (n = 2 or n = 4). The principal quantum. What Is An Orbital Shell In Chemistry.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Is An Orbital Shell In Chemistry The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The principal quantum number, n, can be any positive integer. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. Each atomic orbital can be. What Is An Orbital Shell In Chemistry.
From antranik.org
Electrons shells and orbitals What Is An Orbital Shell In Chemistry The general region for value of energy of the orbital and the average. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The principal quantum number, n, can be any positive integer. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not. What Is An Orbital Shell In Chemistry.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is An Orbital Shell In Chemistry The general region for value of energy of the orbital and the average. The first character indicates the shell (n = 2 or n = 4). The principal quantum number, n, can be any positive integer. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The orbitals in an. What Is An Orbital Shell In Chemistry.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Is An Orbital Shell In Chemistry Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The first character indicates the shell (n = 2 or n = 4). An atomic orbital is characterized by three quantum numbers. The general region for value of energy of the orbital and the average. The orbitals in an atom. What Is An Orbital Shell In Chemistry.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is An Orbital Shell In Chemistry Each atomic orbital can be occupied by a maximum of two electrons. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The electrons in an atom. What Is An Orbital Shell In Chemistry.
From chem.libretexts.org
7.6 The Shape of Atomic Orbitals Chemistry LibreTexts What Is An Orbital Shell In Chemistry An atomic orbital is characterized by three quantum numbers. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered. Each atomic orbital can be occupied by a maximum of two electrons. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from. What Is An Orbital Shell In Chemistry.
From www.britannica.com
Chemical bonding Atomic Orbitals, Shapes, Hybridization Britannica What Is An Orbital Shell In Chemistry The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are. What Is An Orbital Shell In Chemistry.
From klatysxav.blob.core.windows.net
What Is Electron Shell Configuration at Louis Rozier blog What Is An Orbital Shell In Chemistry Each atomic orbital can be occupied by a maximum of two electrons. The first character indicates the shell (n = 2 or n = 4). The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The general region for value of energy of the orbital and the average.. What Is An Orbital Shell In Chemistry.
From guweb2.gonzaga.edu
CHEM 101 Lecture 7 What Is An Orbital Shell In Chemistry Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. The principal quantum number, n, can be any positive integer. An atomic orbital is characterized by three quantum numbers. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the. What Is An Orbital Shell In Chemistry.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is An Orbital Shell In Chemistry Each atomic orbital can be occupied by a maximum of two electrons. The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The general region for value of energy of the orbital and the average. Orbitals exist at specific energy levels and electrons can only be found. What Is An Orbital Shell In Chemistry.
From pressbooks.bccampus.ca
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry What Is An Orbital Shell In Chemistry An atomic orbital is characterized by three quantum numbers. The first character indicates the shell (n = 2 or n = 4). The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The orbitals in an atom are organized into different layers around the nucleus called electron shells,. What Is An Orbital Shell In Chemistry.
From www.thoughtco.com
Electron Configuration Chart What Is An Orbital Shell In Chemistry The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The principal quantum number, n, can be any positive integer. An atomic orbital is characterized. What Is An Orbital Shell In Chemistry.