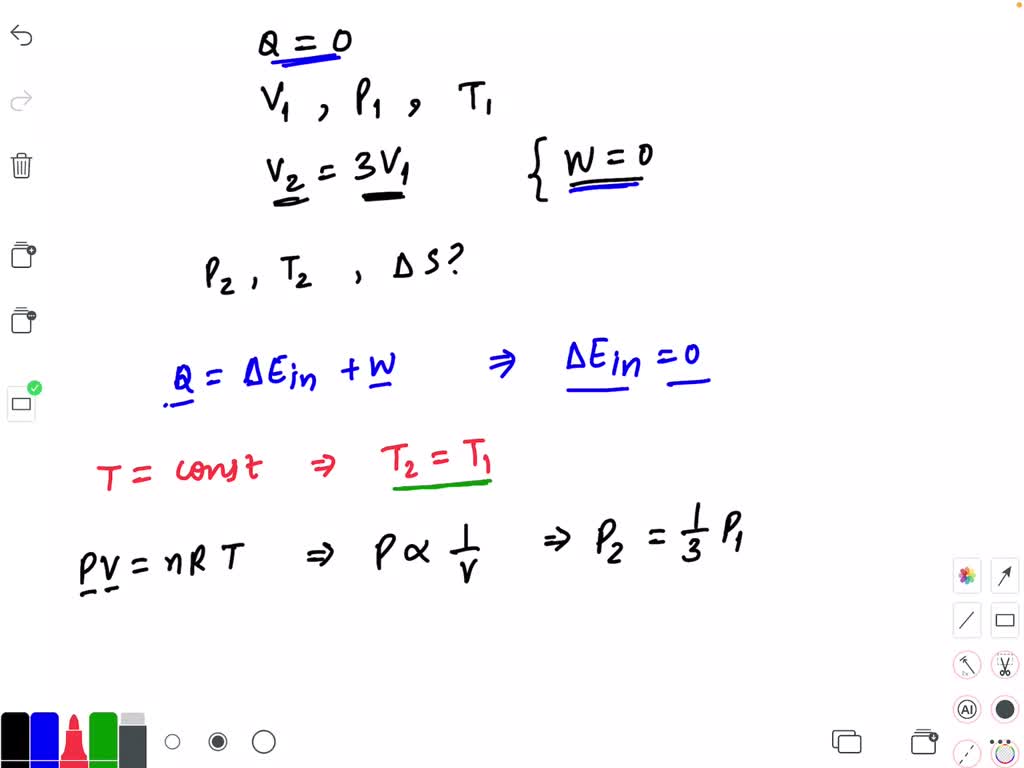
Piston Gas Temperature . The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. The piston coming towards the. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). Let’s consider a case in. common situations that are considered are: when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. heat is being conducted from the left chamber to the right one through the lead piston. No work is done by the gas on its surroundings, nor does. The pressure on the volume gas is constant. if the gas is ideal, the internal energy depends only on the temperature. We are given the rate of heat transfer, the length of the piston, its cross. Therefore, when an ideal gas expands. the piston represents the racket and the gas molecules the tennis balls.
from solvedlib.com
Let’s consider a case in. The pressure on the volume gas is constant. the piston represents the racket and the gas molecules the tennis balls. The piston coming towards the. Therefore, when an ideal gas expands. heat is being conducted from the left chamber to the right one through the lead piston. No work is done by the gas on its surroundings, nor does. common situations that are considered are: when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ).
An ideal gas is placed in a cylinder with a sliding p… SolvedLib
Piston Gas Temperature Let’s consider a case in. common situations that are considered are: the piston represents the racket and the gas molecules the tennis balls. We are given the rate of heat transfer, the length of the piston, its cross. The piston coming towards the. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. No work is done by the gas on its surroundings, nor does. The pressure on the volume gas is constant. Therefore, when an ideal gas expands. Let’s consider a case in. if the gas is ideal, the internal energy depends only on the temperature. heat is being conducted from the left chamber to the right one through the lead piston.
From www.toppr.com
One mole of an ideal gas is contained in a cylinder with a movable Piston Gas Temperature The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). The piston coming towards the. the piston represents the racket and the gas molecules the tennis balls. when the. Piston Gas Temperature.
From www.numerade.com
SOLVED 3.13. Work and Heat A fixed mass m of carbon monoxide (CO) gas Piston Gas Temperature if the gas is ideal, the internal energy depends only on the temperature. heat is being conducted from the left chamber to the right one through the lead piston. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. the piston represents. Piston Gas Temperature.
From www.chegg.com
Solved A closed pistoncylinder device contains a gas Piston Gas Temperature common situations that are considered are: No work is done by the gas on its surroundings, nor does. Therefore, when an ideal gas expands. The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is. Piston Gas Temperature.
From www.chegg.com
Solved (1) Consider 1mol of an ideal gas in a Piston Gas Temperature for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). Therefore, when an ideal gas expands. The piston coming towards the. the piston represents the racket and the gas molecules the tennis balls. when the gas temperature reaches 90°c, the piston is at a. Piston Gas Temperature.
From dxomwvret.blob.core.windows.net
Gas Turbine Engine Vs Piston Engine at Misty Feeney blog Piston Gas Temperature The pressure on the volume gas is constant. heat is being conducted from the left chamber to the right one through the lead piston. Therefore, when an ideal gas expands. We are given the rate of heat transfer, the length of the piston, its cross. if the gas is ideal, the internal energy depends only on the temperature.. Piston Gas Temperature.
From www.researchgate.net
Temperature distribution in the piston Download Scientific Diagram Piston Gas Temperature heat is being conducted from the left chamber to the right one through the lead piston. if the gas is ideal, the internal energy depends only on the temperature. The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. Let’s consider a case in. We are given the rate of heat transfer, the. Piston Gas Temperature.
From www.researchgate.net
Temperature distribution of the engine piston [ o C] without (upper Piston Gas Temperature Therefore, when an ideal gas expands. The pressure on the volume gas is constant. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. common situations that are considered are: heat is being conducted from the left chamber to the right one through. Piston Gas Temperature.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica Piston Gas Temperature The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. heat is being conducted from the left chamber to the right one through the lead piston. The piston coming towards the. common situations that are considered are: No work is done by the gas on its surroundings, nor does. Let’s consider a case. Piston Gas Temperature.
From www.researchgate.net
Temperature distribution in the piston Download Scientific Diagram Piston Gas Temperature Let’s consider a case in. No work is done by the gas on its surroundings, nor does. The piston coming towards the. common situations that are considered are: Therefore, when an ideal gas expands. The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. We are given the rate of heat transfer, the length. Piston Gas Temperature.
From www.researchgate.net
Temperature distribution on the surfaces ZrO 2 coated piston (a,c,d is Piston Gas Temperature No work is done by the gas on its surroundings, nor does. We are given the rate of heat transfer, the length of the piston, its cross. Therefore, when an ideal gas expands. Let’s consider a case in. The piston coming towards the. The pressure on the volume gas is constant. if the gas is ideal, the internal energy. Piston Gas Temperature.
From philschatz.com
The First Law of Thermodynamics and Some Simple Processes · Physics Piston Gas Temperature for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. The pressure on the volume gas is constant. The piston coming towards the. the piston represents the racket and the. Piston Gas Temperature.
From physicslabs.ccnysites.cuny.edu
Ideal Gas Tutorial Piston Gas Temperature for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). Let’s consider a case in. heat is being conducted from the left chamber to the right one through the lead piston. when the gas temperature reaches 90°c, the piston is at a new equilibrium. Piston Gas Temperature.
From www.numerade.com
SOLVED Assume that you have a sample of gas in a cylinder with 3 Piston Gas Temperature heat is being conducted from the left chamber to the right one through the lead piston. The pressure on the volume gas is constant. if the gas is ideal, the internal energy depends only on the temperature. Therefore, when an ideal gas expands. The piston coming towards the. the piston represents the racket and the gas molecules. Piston Gas Temperature.
From www.chegg.com
Solved Problem 3 A pistoncylinder device contains 1.2 kg Piston Gas Temperature The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. heat is being conducted from the left chamber to the right one through the lead piston. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). The pressure on the. Piston Gas Temperature.
From www.chegg.com
Solved 2. 1.50 moles of an ideal gas are held in a cylinder Piston Gas Temperature if the gas is ideal, the internal energy depends only on the temperature. The piston coming towards the. common situations that are considered are: No work is done by the gas on its surroundings, nor does. the piston represents the racket and the gas molecules the tennis balls. We are given the rate of heat transfer, the. Piston Gas Temperature.
From pressbooks.bccampus.ca
4.3 Work Introduction to Engineering Thermodynamics Piston Gas Temperature No work is done by the gas on its surroundings, nor does. Let’s consider a case in. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. if the gas is ideal, the internal energy depends only on the temperature. Therefore, when an ideal. Piston Gas Temperature.
From www.toppr.com
A gas is enclosed in a cylinder with a movable frictionless piston. Its Piston Gas Temperature for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). heat is being conducted from the left chamber to the right one through the lead piston. We are given the rate of heat transfer, the length of the piston, its cross. if the gas. Piston Gas Temperature.
From www.youtube.com
2.59 As shown in Fig. P2.59, a gas contained within a pistoncylinder Piston Gas Temperature when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. the piston represents the racket and the gas molecules the tennis balls. Therefore, when an ideal gas expands. The pressure on the volume gas is constant. Let’s consider a case in. The piston coming. Piston Gas Temperature.
From www.tec-science.com
Why do pressure and temperature increase during the compression of a Piston Gas Temperature Therefore, when an ideal gas expands. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. if the gas is. Piston Gas Temperature.
From www.researchgate.net
Temperature on gas exposed surface piston with calculated combustion Piston Gas Temperature Let’s consider a case in. if the gas is ideal, the internal energy depends only on the temperature. The pressure on the volume gas is constant. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. The piston coming towards the. heat is. Piston Gas Temperature.
From exojzprjf.blob.core.windows.net
How To Pistons Work at Laurie Kasten blog Piston Gas Temperature when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. No work is done by the gas on its surroundings, nor does. common situations that are considered are: if the gas is ideal, the internal energy depends only on the temperature. The ideal. Piston Gas Temperature.
From www.researchgate.net
Temperature distribution in the piston Download Scientific Diagram Piston Gas Temperature No work is done by the gas on its surroundings, nor does. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). Let’s consider a case in. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in. Piston Gas Temperature.
From courses.lumenlearning.com
The Theory CHEM 1305 General Chemistry I—Lecture Piston Gas Temperature Let’s consider a case in. if the gas is ideal, the internal energy depends only on the temperature. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. The piston coming towards the. the piston represents the racket and the gas molecules the. Piston Gas Temperature.
From www.researchgate.net
Temperature distribution on the surface of the piston Download Piston Gas Temperature No work is done by the gas on its surroundings, nor does. The piston coming towards the. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. heat is being conducted from the left chamber to the right one through the lead piston. The. Piston Gas Temperature.
From slideplayer.com
Engineering Thermodynamics ppt download Piston Gas Temperature The piston coming towards the. Therefore, when an ideal gas expands. Let’s consider a case in. The pressure on the volume gas is constant. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. heat is being conducted from the left chamber to the. Piston Gas Temperature.
From www.coursehero.com
[Solved] Air in a pistoncylinder assembly and modeled as an ideal gas Piston Gas Temperature The pressure on the volume gas is constant. The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. common situations that are considered are: if the gas is ideal, the internal energy depends only on the temperature. for an ideal gas, this means the volume of a gas is proportional to its. Piston Gas Temperature.
From www.researchgate.net
Exhaust gas temperature at measurement point 1 as a function of Piston Gas Temperature The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. The piston coming towards the. We are given the rate of heat transfer, the length of the piston, its cross. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as.. Piston Gas Temperature.
From physics.stackexchange.com
thermodynamics How to find work done by heated gas and what causes Piston Gas Temperature The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. if the gas is ideal, the internal energy depends only on the temperature. No work is done by the gas on its surroundings, nor does. Therefore, when an ideal gas expands. The pressure on the volume gas is constant. common situations that are. Piston Gas Temperature.
From www.numerade.com
SOLVED Question 2 points Save Answer 4 frictionless gasfilled Piston Gas Temperature when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. Let’s consider a case in. Therefore, when an ideal gas expands. if the gas is ideal, the internal energy. Piston Gas Temperature.
From www.chegg.com
Solved 4. As shown, a gas contained within a pistoncylinder Piston Gas Temperature The piston coming towards the. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in the cylinder is the same as. for an ideal gas, this means the volume of a gas is proportional to its temperature (historically, this is called charles’ law ). the piston represents the racket. Piston Gas Temperature.
From courses.lumenlearning.com
Introduction Boundless Physics Piston Gas Temperature common situations that are considered are: if the gas is ideal, the internal energy depends only on the temperature. The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. The pressure on the volume gas is constant. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but. Piston Gas Temperature.
From www.researchgate.net
Temperature distribution (°C) of the uncoated (conventional) piston Piston Gas Temperature The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. heat is being conducted from the left chamber to the right one through the lead piston. No work is done by the gas on its surroundings, nor does. The piston coming towards the. the piston represents the racket and the gas molecules the. Piston Gas Temperature.
From solvedlib.com
An ideal gas is placed in a cylinder with a sliding p… SolvedLib Piston Gas Temperature heat is being conducted from the left chamber to the right one through the lead piston. common situations that are considered are: The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal. if the gas is ideal, the internal energy depends only on the temperature. We are given the rate of heat. Piston Gas Temperature.
From www.doubtnut.com
A gas is enclosed in a cylindrical can fitted with a piston. The walls Piston Gas Temperature Therefore, when an ideal gas expands. heat is being conducted from the left chamber to the right one through the lead piston. if the gas is ideal, the internal energy depends only on the temperature. the piston represents the racket and the gas molecules the tennis balls. The piston coming towards the. No work is done by. Piston Gas Temperature.
From www.chegg.com
Solved A gas is confined in a cylinder by a piston. The Piston Gas Temperature The piston coming towards the. We are given the rate of heat transfer, the length of the piston, its cross. The pressure on the volume gas is constant. the piston represents the racket and the gas molecules the tennis balls. when the gas temperature reaches 90°c, the piston is at a new equilibrium position, but the pressure in. Piston Gas Temperature.