What Means Latent Heat Of Vaporization . because this energy enters or leaves a system during a phase change without causing a temperature change in the. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. latent heat of vaporization: latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. That associated with vaporizing a liquid or a. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion;
from ar.inspiredpencil.com
the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; That associated with vaporizing a liquid or a. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. because this energy enters or leaves a system during a phase change without causing a temperature change in the. latent heat of vaporization: latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert.
Images Of Vaporization
What Means Latent Heat Of Vaporization the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. because this energy enters or leaves a system during a phase change without causing a temperature change in the. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. That associated with vaporizing a liquid or a. latent heat of vaporization: the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion;
From www.chegg.com
Solved What is the latent heat of vaporization for water at What Means Latent Heat Of Vaporization because this energy enters or leaves a system during a phase change without causing a temperature change in the. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. That associated with vaporizing a liquid or a. latent heat of vaporization: the latent heat of vaporization δh corresponds. What Means Latent Heat Of Vaporization.
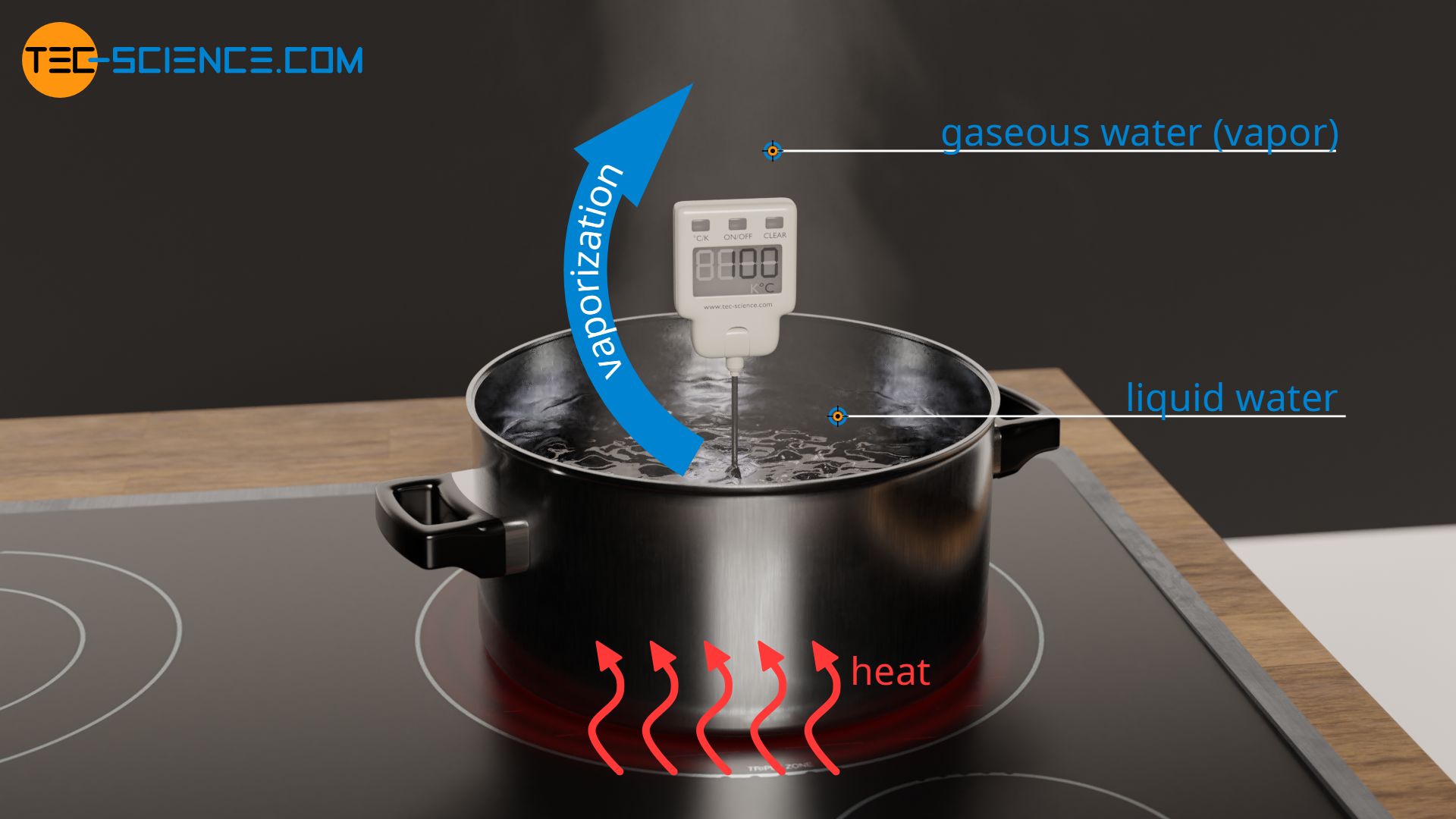
From www.tec-science.com
Specific latent heat of vaporization tecscience What Means Latent Heat Of Vaporization because this energy enters or leaves a system during a phase change without causing a temperature change in the. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. That associated. What Means Latent Heat Of Vaporization.
From www.youtube.com
Latent Heat Of Vaporization 10 (Hindi) YouTube What Means Latent Heat Of Vaporization That associated with vaporizing a liquid or a. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. because this energy enters or leaves a system during a phase change without causing a temperature change in the. latent heat of vaporization: the latent heat of vaporization δh corresponds. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT Change of State PowerPoint Presentation, free download ID1562618 What Means Latent Heat Of Vaporization because this energy enters or leaves a system during a phase change without causing a temperature change in the. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download What Means Latent Heat Of Vaporization The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. That associated with vaporizing a liquid or a. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. the latent heat associated with melting a solid or freezing a liquid. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT Master Streams PowerPoint Presentation, free download ID1597647 What Means Latent Heat Of Vaporization latent heat of vaporization: because this energy enters or leaves a system during a phase change without causing a temperature change in the. That associated with vaporizing a liquid or a. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. latent heat of vaporization. What Means Latent Heat Of Vaporization.
From www.pinterest.com
Heat Of Vaporization Easy Science Easy science, Physics concepts What Means Latent Heat Of Vaporization latent heat of vaporization: That associated with vaporizing a liquid or a. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; because this energy enters or leaves a system. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT Phase Changes and their Calculations PowerPoint Presentation What Means Latent Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. because this energy enters or leaves a system during. What Means Latent Heat Of Vaporization.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy What Means Latent Heat Of Vaporization That associated with vaporizing a liquid or a. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; The latent heat of vaporization is the heat absorbed or released when matter vaporizes,. What Means Latent Heat Of Vaporization.
From www.tec-science.com
Specific latent heat of vaporization tecscience What Means Latent Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. That associated with vaporizing a liquid or a. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. the latent heat associated. What Means Latent Heat Of Vaporization.
From ar.inspiredpencil.com
Heat Of Vaporization Equation What Means Latent Heat Of Vaporization The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. the latent heat associated with melting a solid or freezing a liquid is called the heat of. What Means Latent Heat Of Vaporization.
From material-properties.org
Latent Heat of Vaporization of Chemical Elements Material Properties What Means Latent Heat Of Vaporization the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT Phase of Water and Latent Heats PowerPoint Presentation, free What Means Latent Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. latent heat of vaporization: the latent heat of vaporization δh corresponds. What Means Latent Heat Of Vaporization.
From fer-io.blogspot.com
Specific Latent Heat Of Vaporisation Formula Specific Latent Heat What Means Latent Heat Of Vaporization That associated with vaporizing a liquid or a. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the latent heat. What Means Latent Heat Of Vaporization.
From www.tec-science.com
Specific latent heat of condensation tecscience What Means Latent Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; The latent heat of vaporization is the heat absorbed or released when matter. What Means Latent Heat Of Vaporization.
From www.youtube.com
"Understanding Sensible Heat vs. Latent Heat What's the Difference What Means Latent Heat Of Vaporization latent heat of vaporization: the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat of vaporization δh corresponds to the amount of energy that must be. What Means Latent Heat Of Vaporization.
From byjus.com
Draw a labelled diagram of the apparatus you would use to determine the What Means Latent Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. That associated with vaporizing a liquid or a. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; because this energy enters. What Means Latent Heat Of Vaporization.
From www.sliderbase.com
Heat of Fusion What Means Latent Heat Of Vaporization the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. because this energy enters or leaves a system during a phase change without causing a temperature change in. What Means Latent Heat Of Vaporization.
From www.tec-science.com
Specific latent heat of condensation tecscience What Means Latent Heat Of Vaporization because this energy enters or leaves a system during a phase change without causing a temperature change in the. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; latent heat of vaporization: The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase. What Means Latent Heat Of Vaporization.
From www.numerade.com
SOLVED a) What is meant by the word 'Latent' in latent heat.b) Explain What Means Latent Heat Of Vaporization because this energy enters or leaves a system during a phase change without causing a temperature change in the. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. latent heat of vaporization is the heat consumed or discharged when. What Means Latent Heat Of Vaporization.
From ar.inspiredpencil.com
Images Of Vaporization What Means Latent Heat Of Vaporization latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. because this energy enters or leaves a system during a phase change without causing a temperature change in the. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; The latent. What Means Latent Heat Of Vaporization.
From www.researchgate.net
Latent heat of vaporization as a function of (a) salinity (at 20 °C and What Means Latent Heat Of Vaporization latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to. What Means Latent Heat Of Vaporization.
From www.nachi.org
Latent Heat of Vaporization Inspection Gallery InterNACHI® What Means Latent Heat Of Vaporization the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; That associated with vaporizing a liquid or a. because this energy enters or leaves a system during a. What Means Latent Heat Of Vaporization.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo What Means Latent Heat Of Vaporization the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; latent heat of vaporization: the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. That associated with vaporizing a liquid or a.. What Means Latent Heat Of Vaporization.
From www.ck12.org
Heats of Vaporization and Condensation CK12 Foundation What Means Latent Heat Of Vaporization latent heat of vaporization: the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. That associated with vaporizing a liquid or a. because this energy enters or. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT Latent Heat PowerPoint Presentation, free download ID5759776 What Means Latent Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat associated with melting a solid or freezing a. What Means Latent Heat Of Vaporization.
From giungiun.com
What Is Specific Latent Heat Unlocking Its Mysteries What Means Latent Heat Of Vaporization latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. because this energy enters or leaves a system during a phase change without causing a temperature change in the. . What Means Latent Heat Of Vaporization.
From www.tec-science.com
Specific latent heat of vaporization tecscience What Means Latent Heat Of Vaporization The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. because this energy enters or leaves a system during a phase change without causing a temperature change in the. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system. What Means Latent Heat Of Vaporization.
From www.youtube.com
Measuring the specific latent heat of vaporization of water YouTube What Means Latent Heat Of Vaporization because this energy enters or leaves a system during a phase change without causing a temperature change in the. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system. What Means Latent Heat Of Vaporization.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Means Latent Heat Of Vaporization because this energy enters or leaves a system during a phase change without causing a temperature change in the. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system. What Means Latent Heat Of Vaporization.
From ar.inspiredpencil.com
Heat Of Vaporization Equation What Means Latent Heat Of Vaporization latent heat of vaporization: That associated with vaporizing a liquid or a. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. the latent heat associated. What Means Latent Heat Of Vaporization.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy What Means Latent Heat Of Vaporization That associated with vaporizing a liquid or a. latent heat of vaporization: the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. latent heat of vaporization is the heat. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID5769198 What Means Latent Heat Of Vaporization latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. The latent heat of vaporization is the heat absorbed or released when matter vaporizes, changing phase from liquid to gas. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; latent. What Means Latent Heat Of Vaporization.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID8874124 What Means Latent Heat Of Vaporization latent heat of vaporization: because this energy enters or leaves a system during a phase change without causing a temperature change in the. the latent heat of vaporization δh corresponds to the amount of energy that must be supplied to the system to convert. the latent heat associated with melting a solid or freezing a liquid. What Means Latent Heat Of Vaporization.
From www.bartleby.com
Answered Latent heats of vaporization at 25°C in… bartleby What Means Latent Heat Of Vaporization latent heat of vaporization is the heat consumed or discharged when matter disintegrates, changing state from fluid to. the latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to. What Means Latent Heat Of Vaporization.