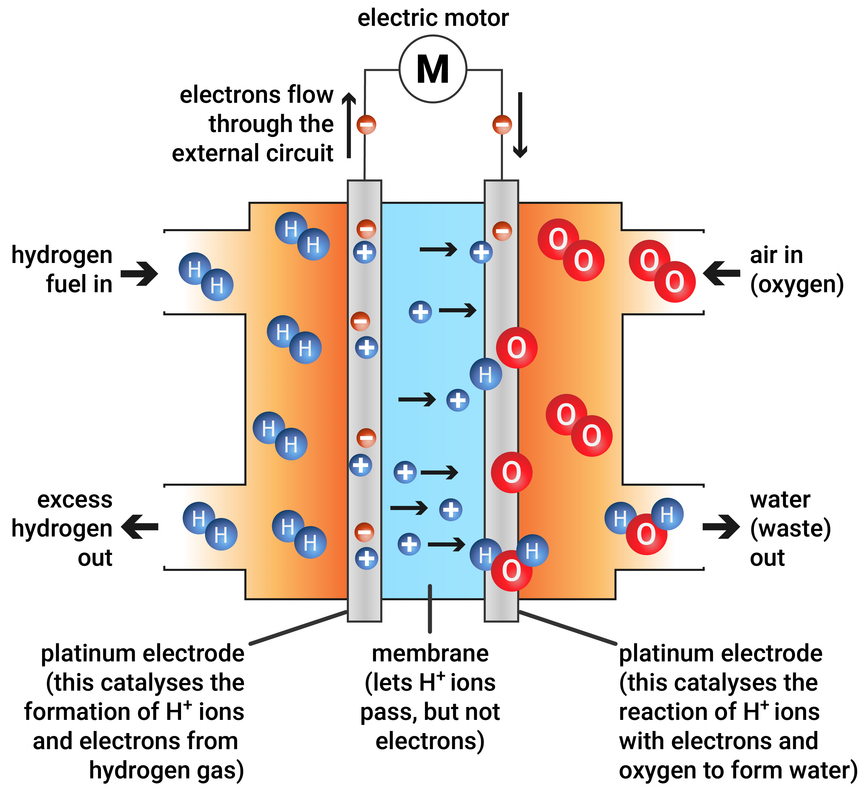
Fuel Cell Chemical . A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. If hydrogen is the fuel, the only. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. Fuel cells are classified primarily by the kind of electrolyte they employ. Fuel cells have an important advantage over all.
from revisechemistry.uk
A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. Fuel cells are classified primarily by the kind of electrolyte they employ. If hydrogen is the fuel, the only. Fuel cells have an important advantage over all.
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk
Fuel Cell Chemical A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. If hydrogen is the fuel, the only. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells have an important advantage over all. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells are classified primarily by the kind of electrolyte they employ.
From www.youtube.com
Proton Exchange Membrane Fuel Cell, Introduction, Principle, Advantages Fuel Cell Chemical If hydrogen is the fuel, the only. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. Fuel cells have an important advantage over all. Fuel cells are classified. Fuel Cell Chemical.
From www.researchgate.net
Overview of the various types of fuel cells with the following Fuel Cell Chemical Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including. Fuel Cell Chemical.
From www.tes.com
Chemical Cells and Fuel Cells GCSE AQA Chemistry HIGHER Teaching Fuel Cell Chemical This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. Fuel cells are classified primarily by the kind of electrolyte they employ. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This review discusses the history, fundamentals, and applications of different fuel. Fuel Cell Chemical.
From www.mdpi.com
Energies Free FullText Principles and Materials Aspects of Direct Fuel Cell Chemical Fuel cells have an important advantage over all. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. If hydrogen is the fuel, the only. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. This review discusses the history, fundamentals,. Fuel Cell Chemical.
From www.dreamstime.com
Hydrogen Fuel Cell. Chemical Reaction Producing Electricity. H2 Energy Fuel Cell Chemical Fuel cells are classified primarily by the kind of electrolyte they employ. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cell, any of a class. Fuel Cell Chemical.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Fuel Cell Chemical Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. A fuel cell resembles a battery. Fuel Cell Chemical.
From www.researchgate.net
Operating principle of the Molten Carbonate Fuel Cell when H2 from Fuel Cell Chemical A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. If hydrogen is the fuel, the only. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. Fuel Cell Chemical.
From www.researchgate.net
Application of ALD on fuel cells. a) Different types of fuel cells with Fuel Cell Chemical A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and. Fuel Cell Chemical.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cell Chemical This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. A fuel cell uses the chemical energy of hydrogen. Fuel Cell Chemical.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Fuel Cell Chemical A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cell, any of a class of devices that convert the chemical energy of a fuel. Fuel Cell Chemical.
From chem.libretexts.org
6.5 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cell Chemical This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly. Fuel Cell Chemical.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell Chemical This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells are classified. Fuel Cell Chemical.
From www.researchgate.net
Scheme of a solid oxide fuel cell. Download Scientific Diagram Fuel Cell Chemical A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. If hydrogen is the fuel, the only. Fuel cell, any of a class of devices that convert the chemical energy of a. Fuel Cell Chemical.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cell Chemical A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. If hydrogen is the fuel, the only. Fuel cells have an important advantage over all. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. This paper provides. Fuel Cell Chemical.
From www.alamy.com
Hydrogen fuel cell, A fuel cell is a device that converts the chemical Fuel Cell Chemical A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. Fuel Cell Chemical.
From www.alamy.com
Hydrogen fuel cell, A fuel cell is a device that converts the chemical Fuel Cell Chemical Fuel cells are classified primarily by the kind of electrolyte they employ. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This paper provides a comprehensive review of fuel cell science. Fuel Cell Chemical.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cell Chemical A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells have an important advantage over all. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including. Fuel Cell Chemical.
From www.vectorstock.com
Molten carbonate fuel cells process Royalty Free Vector Fuel Cell Chemical Fuel cells have an important advantage over all. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell consists of two electrodes—a negative electrode (or anode). Fuel Cell Chemical.
From fuelcellpartnership.org
Frequently Asked Questions California Fuel Cell Partnership Fuel Cell Chemical A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen. Fuel Cell Chemical.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell Chemical This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. Fuel cells have an important advantage over all. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell uses the chemical energy of hydrogen or other. Fuel Cell Chemical.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cell Chemical A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. Fuel cells are classified primarily by the kind of electrolyte they employ. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Fuel Cell Chemical.
From www.dataphysics-instruments.com
How measuring contact angles at high temperatures can help develop fuel Fuel Cell Chemical Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells are classified primarily by the kind of electrolyte they employ. A fuel cell like this will. Fuel Cell Chemical.
From www.alamy.com
Fuel cell diagram. Vector. Device that converts chemical potential Fuel Cell Chemical If hydrogen is the fuel, the only. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including. Fuel Cell Chemical.
From chem.libretexts.org
2.2. Hydrogen, The Simplest Atom Chemistry LibreTexts Fuel Cell Chemical A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte.. Fuel Cell Chemical.
From lylagokejames.blogspot.com
Describe How a Fuel Cell Works Fuel Cell Chemical A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell consists of two electrodes—a negative electrode (or. Fuel Cell Chemical.
From www.myelectrical2015.com
Electrical Revolution Fuel Cell Chemical Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or. Fuel Cell Chemical.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cell Chemical This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. Fuel cells are classified primarily by the kind of electrolyte they employ. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. This review discusses the history, fundamentals, and applications of different fuel. Fuel Cell Chemical.
From mungfali.com
Fuel Cell Anode And Cathode Fuel Cell Chemical A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of. Fuel Cell Chemical.
From courses.lumenlearning.com
Applications of Redox Reactions Voltaic Cells Introductory Chemistry Fuel Cell Chemical Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period. Fuel Cell Chemical.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Fuel Cell Chemical This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. If hydrogen is the fuel, the only. A fuel cell like this will continue to operate and produce electrical energy as long. Fuel Cell Chemical.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Cell Chemical A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or. Fuel Cell Chemical.
From www.alamy.com
Fuel cell and Liion battery diagram. Vector. Device that converts Fuel Cell Chemical Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. Fuel cells have an important advantage over all. A fuel cell consists of two electrodes—a negative electrode (or anode). Fuel Cell Chemical.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Fuel Cell Chemical If hydrogen is the fuel, the only. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange membrane fuel cells. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell like this will continue to operate and produce electrical energy as. Fuel Cell Chemical.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Fuel Cell Chemical Fuel cells have an important advantage over all. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. This review discusses the history, fundamentals, and applications of different fuel cell technologies,. Fuel Cell Chemical.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Fuel Cell Chemical A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells are classified primarily by the kind of electrolyte they employ. This paper provides a comprehensive review of fuel cell science and engineering with a focus on hydrogen fuel cells. This review discusses the history, fundamentals, and. Fuel Cell Chemical.