Calorimetry Examples . Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. If the heat capacity of the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction.
from www.studocu.com
If the heat capacity of the. For example, when an exothermic reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change.
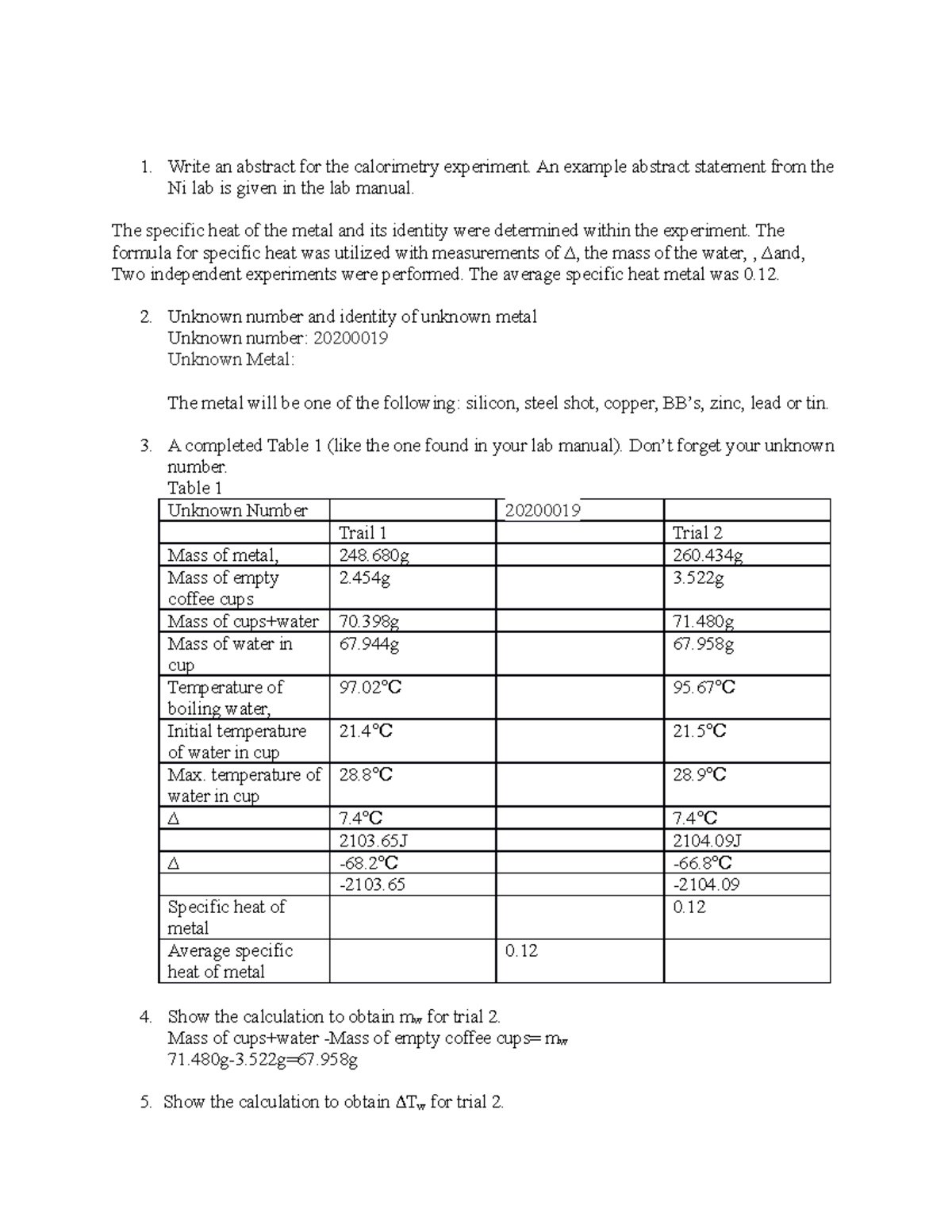
Calorimetry Lab Report Write an abstract for the calorimetry
Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. If the heat capacity of the.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. when 1.00 g. Calorimetry Examples.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Examples apply the first law of thermodynamics to calorimetry. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Compare heat flow from hot to cold objects in an ideal calorimeter. Calorimetry Examples.
From users.highland.edu
Calorimetry Calorimetry Examples Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimetry Examples.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Examples calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Compare heat flow from hot to cold. Calorimetry Examples.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. For example, when an exothermic reaction. If. Calorimetry Examples.
From physique.ensc-rennes.fr
TP calorimetry Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the. Calorimetry Examples.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. If the heat capacity of the. a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimetry Examples.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If the heat capacity of the. apply the first law of thermodynamics to. Calorimetry Examples.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimetry Examples.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. when 1.00 g of coal is burned in a bomb calorimeter, the temperature. Calorimetry Examples.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Examples when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example,. Calorimetry Examples.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Examples For example, when an exothermic reaction. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.. Calorimetry Examples.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. For example, when an exothermic reaction. calorimetry measures enthalpy changes. Calorimetry Examples.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Examples For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure. Calorimetry Examples.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Examples apply the first law of thermodynamics to calorimetry. For example, when an exothermic reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used. Calorimetry Examples.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. calorimetry measures enthalpy changes. Calorimetry Examples.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Examples For example, when an exothermic reaction. For example, when an exothermic reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Examples.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Examples apply the first law of thermodynamics to calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c.. Calorimetry Examples.
From www.pearson.com
Chapter 09 16 Constant Pressure Calorimetry (coffee cup) Channels Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.. Calorimetry Examples.
From www.animalia-life.club
Calorimeter Diagram Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change,. Calorimetry Examples.
From quizunderfires.z21.web.core.windows.net
Units Of Calorimeter Constant Calorimetry Examples apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the. Calorimetry Examples.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Concept Calorimetry Examples apply the first law of thermodynamics to calorimetry. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical. Calorimetry Examples.
From www.carolina.com
Food Calorimetry How to Measure Calories in Food Calorimetry Examples when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate heat,. Calorimetry Examples.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Calorimetry Examples calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. For example, when an exothermic reaction. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to. Calorimetry Examples.
From studylib.net
Calorimetry Calorimetry Examples Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. apply the first law of thermodynamics to calorimetry. If the heat capacity of the. a calorimeter is a device used to. Calorimetry Examples.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Examples Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. For example, when an exothermic reaction. If the heat. Calorimetry Examples.
From valentinashfordlibraries.myshopify.com
example text 1 Bench Scale Calorimetry in Chemical Reaction A Calorimetry Examples when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. For. Calorimetry Examples.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Examples For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. a calorimeter is a device used to measure the amount of heat involved in a. Calorimetry Examples.
From www.youtube.com
Bomb Calorimetry Problem (Chemistry) YouTube Calorimetry Examples Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimetry Examples.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. For example, when an exothermic reaction. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the. Calorimetry Examples.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Examples a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Examples.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Examples For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot. Calorimetry Examples.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Calorimetry Examples when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If the heat capacity of the.. Calorimetry Examples.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Examples If the heat capacity of the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a. Calorimetry Examples.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Examples apply the first law of thermodynamics to calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of. Calorimetry Examples.