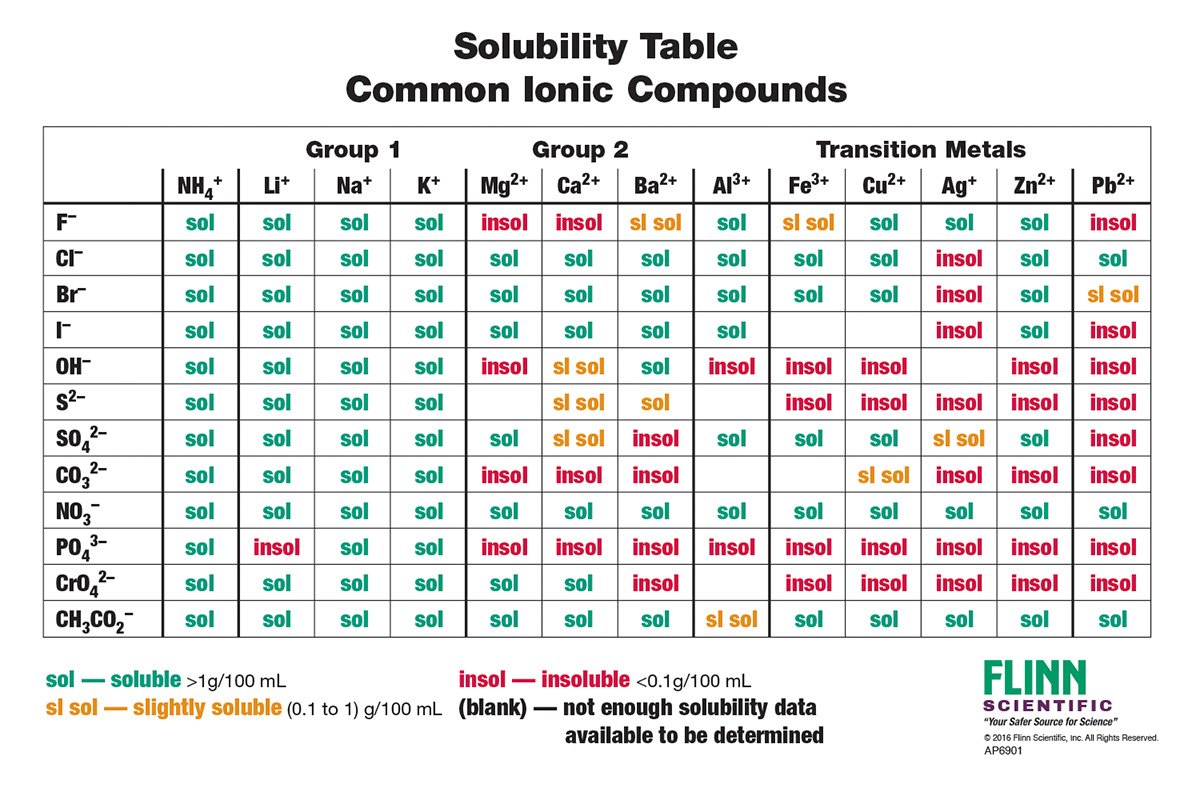
Is Ca Oh 2 Insoluble . It forms when calcium oxide is mixed. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. A saturated solution is an equilibrium, that. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. The oxides of calcium, strontium, and barium. When dissolved in water to a saturation. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water.
from www.flinnsci.ca
ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. A saturated solution is an equilibrium, that. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. When dissolved in water to a saturation. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. It forms when calcium oxide is mixed. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. The oxides of calcium, strontium, and barium.
Solubility Rules Charts for Chemistry
Is Ca Oh 2 Insoluble ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. A saturated solution is an equilibrium, that. The oxides of calcium, strontium, and barium. When dissolved in water to a saturation. It forms when calcium oxide is mixed. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry.
From www.youtube.com
How to Write the Net Ionic Equation for Ca(OH)2 + CO2 = CaCO3 + H2O Is Ca Oh 2 Insoluble is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. The oxides of calcium, strontium, and barium. It forms when calcium oxide is mixed. mgo. Is Ca Oh 2 Insoluble.
From maternityandbabyshoppingmart.com
6+ Fresh Solubility And Concentration Worksheet Mate Template Design Is Ca Oh 2 Insoluble calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. The oxides of calcium, strontium, and barium. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. ca (oh) 2 is quite soluble in glycerol and acids, but. Is Ca Oh 2 Insoluble.
From www.chegg.com
Solved A. Soluble and Insoluble Salts 4. Compounds in 1. Is Ca Oh 2 Insoluble ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. It forms when calcium oxide is mixed. When dissolved in water to a saturation. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry.. Is Ca Oh 2 Insoluble.
From retromania.cz
Sea slug controller Perforate calcium carbonate heated reaction Is Ca Oh 2 Insoluble is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. calcium hydroxide,. Is Ca Oh 2 Insoluble.
From www.youtube.com
Is Ca(NO3)2 Soluble or Insoluble in Water? YouTube Is Ca Oh 2 Insoluble is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. ca (oh) 2 is quite soluble in glycerol and acids, but. Is Ca Oh 2 Insoluble.
From www.nagwa.com
Question Video Determining Which Reaction Will Produce a Precipitate Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. When dissolved in water to a saturation. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. mgo is basic. Is Ca Oh 2 Insoluble.
From www.teachoo.com
Combination Reaction Definition with 4 Examples Class 10 Chemistry Is Ca Oh 2 Insoluble mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. It forms when calcium oxide is mixed. The oxides of calcium, strontium, and barium. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. is ca(oh)2 (calcium hydroxide) soluble or insoluble. Is Ca Oh 2 Insoluble.
From www.pinterest.com
Solubility Rules Chart and Memorization Tips How to memorize things Is Ca Oh 2 Insoluble mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. When dissolved in water to a saturation. The oxides of calcium, strontium, and barium. A saturated solution is an equilibrium, that. ca (oh) 2 is. Is Ca Oh 2 Insoluble.
From quizlet.com
Solubility Diagram Quizlet Is Ca Oh 2 Insoluble is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. A saturated solution is an equilibrium, that. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. It forms when calcium oxide is mixed. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. . Is Ca Oh 2 Insoluble.
From www.transtutors.com
(Get Answer) D. Dissolving Insoluble Solids I. Observations On Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. The oxides of calcium, strontium, and barium. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g. Is Ca Oh 2 Insoluble.
From competeipl.com
Natural GAINZ Magazine Fiber Soluble Vs. Insoluble Natural Is Ca Oh 2 Insoluble When dissolved in water to a saturation. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. It forms when calcium oxide is mixed. calcium hydroxide (ca (oh)2), a soft white powder that is widely. Is Ca Oh 2 Insoluble.
From www.youtube.com
How to Write the Net Ionic Equation for HNO3 + Ca(OH)2 YouTube Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. When dissolved in water to a saturation. A saturated solution is an equilibrium, that. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. It forms when calcium oxide is mixed. . Is Ca Oh 2 Insoluble.
From www.homeworklib.com
Classify each of the following substances as either a strong or weak Is Ca Oh 2 Insoluble calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. When dissolved in water to a saturation. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. A saturated solution is an equilibrium, that. is ca(oh)2 (calcium hydroxide) soluble or insoluble in. Is Ca Oh 2 Insoluble.
From www.pathwaystochemistry.com
Precipitation Reactions Pathways to Chemistry Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. calcium hydroxide, ca (oh) 2, is an ionic solid that. Is Ca Oh 2 Insoluble.
From www.answersarena.com
[Solved] Select the ionic compound which is insoluble in w Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. It forms when calcium oxide is mixed. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g. Is Ca Oh 2 Insoluble.
From www.jianghutio2.com
High Purity Ca(OH)2 For Calcium Stearate PVC Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. A saturated solution is an equilibrium, that. When dissolved in water to a saturation. calcium hydroxide, ca (oh) 2, is an. Is Ca Oh 2 Insoluble.
From www.pinterest.es
Insoluble Fiber Fiber Nutrition, Nutrition Chart Is Ca Oh 2 Insoluble It forms when calcium oxide is mixed. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. When dissolved in water to a saturation. The oxides of calcium, strontium, and barium. A saturated. Is Ca Oh 2 Insoluble.
From www.transtutors.com
(Solved) Predict Whether Ni(OH)2 Should Be Soluble Or Insoluble In Is Ca Oh 2 Insoluble The oxides of calcium, strontium, and barium. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. When dissolved in water to a saturation. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh). Is Ca Oh 2 Insoluble.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Is Ca Oh 2 Insoluble mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. It forms when calcium oxide is mixed. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. ca (oh). Is Ca Oh 2 Insoluble.
From www.slideserve.com
PPT Predicting Products The Activity Series & Solubility Rules Is Ca Oh 2 Insoluble The oxides of calcium, strontium, and barium. It forms when calcium oxide is mixed. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. When dissolved in water to a saturation. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. ca (oh) 2 is quite soluble in glycerol and acids, but. Is Ca Oh 2 Insoluble.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Is Ca Oh 2 Insoluble ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. The oxides of calcium, strontium, and barium. It forms when calcium oxide is mixed. mgo is basic. Is Ca Oh 2 Insoluble.
From courses.lumenlearning.com
Solubility Chemistry for Majors Is Ca Oh 2 Insoluble It forms when calcium oxide is mixed. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. The oxides of calcium, strontium, and barium. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in. Is Ca Oh 2 Insoluble.
From www.youtube.com
Is Ca(OH)2 Soluble or Insoluble in Water? YouTube Is Ca Oh 2 Insoluble calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. When dissolved in water to a. Is Ca Oh 2 Insoluble.
From www.chegg.com
Solved Determining the Ksp of Calcium Hydroxide INTRODUCTION Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. It forms when calcium oxide is mixed. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in. Is Ca Oh 2 Insoluble.
From www.numerade.com
SOLVED a) Predict the sign of ΔS for the dissolution of calcium Is Ca Oh 2 Insoluble mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. It forms when calcium oxide is mixed. calcium hydroxide, ca (oh) 2, is an ionic solid that. Is Ca Oh 2 Insoluble.
From studylib.net
Chapter 15 Ksp Ca(OH)2 Lab Is Ca Oh 2 Insoluble ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. It forms when calcium oxide is mixed. When dissolved in water to a saturation. The oxides of calcium, strontium, and barium. mgo is basic and mg (oh)2 is weakly basic and. Is Ca Oh 2 Insoluble.
From www.alibaba.com
Slaked Lime Ca(oh)2 Calcium Hydroxide 1305620 Powder Food Grade Price Is Ca Oh 2 Insoluble The oxides of calcium, strontium, and barium. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. A saturated solution is an equilibrium, that. It forms when calcium oxide is mixed. is ca(oh)2 (calcium hydroxide) soluble. Is Ca Oh 2 Insoluble.
From www.slideshare.net
8.1 (c) insoluble salts Is Ca Oh 2 Insoluble mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. A saturated solution is an equilibrium, that. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g. Is Ca Oh 2 Insoluble.
From entonionflores.blogspot.com
Ca Oh 2 Equilibrium Equation entonionFlores Is Ca Oh 2 Insoluble ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. When. Is Ca Oh 2 Insoluble.
From www.numerade.com
SOLVED Text Solubility of Ionic Compounds Consult the solubility Is Ca Oh 2 Insoluble mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution called lime water. The oxides of calcium, strontium, and barium. ca (oh) 2 is quite soluble in glycerol and acids,. Is Ca Oh 2 Insoluble.
From www.chegg.com
Solved Scoring Your score will be based on the number of Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. The oxides of calcium, strontium, and barium. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. ca (oh) 2. Is Ca Oh 2 Insoluble.
From www.tessshebaylo.com
Chemical Equation For Calcium Hydroxide Dissolving In Water Tessshebaylo Is Ca Oh 2 Insoluble A saturated solution is an equilibrium, that. calcium hydroxide, ca (oh) 2, is an ionic solid that is slightly soluble in water. The oxides of calcium, strontium, and barium. It forms when calcium oxide is mixed. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. ca (oh) 2 is quite soluble in glycerol and acids, but. Is Ca Oh 2 Insoluble.
From www.youtube.com
How to Write the Name for Ca(OH)2 YouTube Is Ca Oh 2 Insoluble When dissolved in water to a saturation. is ca(oh)2 (calcium hydroxide) soluble or insoluble in water ?. It forms when calcium oxide is mixed. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. The oxides of calcium, strontium, and barium. mgo is basic and mg (oh)2 is weakly basic and. Is Ca Oh 2 Insoluble.
From www.youtube.com
How to Balance Ca(OH)2 + CO2 = CaCO3 + H2O (Limewater plus Carbon Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. mgo is basic and mg (oh)2 is weakly basic and do not dissolve in naoh solution. ca (oh) 2 is only slightly soluble in water (0.16g ca (oh) 2 /100g water at 20°c) forming a basic solution. Is Ca Oh 2 Insoluble.
From geekynutritionist.com
What does insoluble fibre do for you? Is Ca Oh 2 Insoluble calcium hydroxide (ca (oh)2), a soft white powder that is widely used as a raw material in the chemical industry. ca (oh) 2 is quite soluble in glycerol and acids, but only slightly soluble in water. When dissolved in water to a saturation. A saturated solution is an equilibrium, that. mgo is basic and mg (oh)2 is. Is Ca Oh 2 Insoluble.