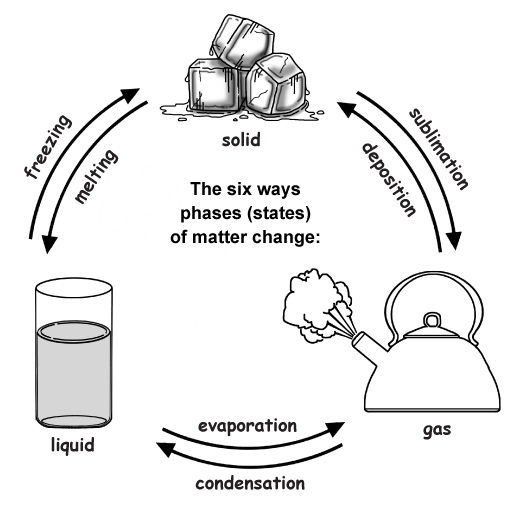
What Is The Phase Change From Liquid To Solid . We take advantage of changes between the gas,. When the rate of condensation becomes equal to the rate of. to calculate the energy changes that accompany phase changes. Melting (solid → liquid) paul taylor / getty images. the change from the gas phase to the liquid is called condensation. This example shows an ice cube melting into. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. there are six ways a substance can change between these three phases; phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,.
from www.exploringnature.org
the change from the gas phase to the liquid is called condensation. to calculate the energy changes that accompany phase changes. there are six ways a substance can change between these three phases; When the rate of condensation becomes equal to the rate of. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. We take advantage of changes between the gas,. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Melting (solid → liquid) paul taylor / getty images. This example shows an ice cube melting into. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma.
Phases of Matter Gas, Liquids, Solids
What Is The Phase Change From Liquid To Solid phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. to calculate the energy changes that accompany phase changes. there are six ways a substance can change between these three phases; When the rate of condensation becomes equal to the rate of. the change from the gas phase to the liquid is called condensation. This example shows an ice cube melting into. Melting (solid → liquid) paul taylor / getty images. We take advantage of changes between the gas,. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,.
From www.vrogue.co
How Does Temperature Affect Solids Liquids And Gases vrogue.co What Is The Phase Change From Liquid To Solid Melting (solid → liquid) paul taylor / getty images. to calculate the energy changes that accompany phase changes. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. This example shows an ice cube. What Is The Phase Change From Liquid To Solid.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids What Is The Phase Change From Liquid To Solid Melting (solid → liquid) paul taylor / getty images. there are six ways a substance can change between these three phases; sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. the change. What Is The Phase Change From Liquid To Solid.
From printablefullchiff.z19.web.core.windows.net
Properties Of Matter Worksheets For Grade 3 What Is The Phase Change From Liquid To Solid When the rate of condensation becomes equal to the rate of. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. sublimation (solid → gas). What Is The Phase Change From Liquid To Solid.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk What Is The Phase Change From Liquid To Solid phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. there are six ways a substance can change between these three phases; This example shows an ice cube melting into. the change from the gas phase to the liquid is called condensation. sublimation (solid →. What Is The Phase Change From Liquid To Solid.
From scientifictutor.org
Chem Definitions of Transition Between States of Matter Scientific What Is The Phase Change From Liquid To Solid We take advantage of changes between the gas,. This example shows an ice cube melting into. there are six ways a substance can change between these three phases; sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. When the rate of condensation becomes equal to the rate of. to calculate the energy changes that. What Is The Phase Change From Liquid To Solid.
From diagramlibrarybecharm.z19.web.core.windows.net
Tv And Pv Diagrams What Is The Phase Change From Liquid To Solid phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. We take advantage of changes between the gas,. there are six ways a substance can change between these three phases; When the rate of condensation becomes equal to the rate of. use of one term or. What Is The Phase Change From Liquid To Solid.
From ny1.com
The amount of water on Earth is a constant What Is The Phase Change From Liquid To Solid When the rate of condensation becomes equal to the rate of. to calculate the energy changes that accompany phase changes. the change from the gas phase to the liquid is called condensation. there are six ways a substance can change between these three phases; phase changes are physical changes that take place when matter changes energy. What Is The Phase Change From Liquid To Solid.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii What Is The Phase Change From Liquid To Solid to calculate the energy changes that accompany phase changes. This example shows an ice cube melting into. We take advantage of changes between the gas,. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. When the rate of condensation becomes equal to the rate of. Melting. What Is The Phase Change From Liquid To Solid.
From www.ebay.com
12 Position Lab Solid Liquid Phase Extraction SPE Manifold Vacuum What Is The Phase Change From Liquid To Solid the change from the gas phase to the liquid is called condensation. there are six ways a substance can change between these three phases; to calculate the energy changes that accompany phase changes. When the rate of condensation becomes equal to the rate of. This example shows an ice cube melting into. We take advantage of changes. What Is The Phase Change From Liquid To Solid.
From www.dreamstime.com
Phase Changes Vector Illustration. Labeled Matter Scheme with Enthalpy What Is The Phase Change From Liquid To Solid use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. We take advantage of changes between the gas,. sublimation (solid → gas) ionization (gas →. What Is The Phase Change From Liquid To Solid.
From platoesg.com
GM Might Cool EV Charge Ports Like Microchips What Is The Phase Change From Liquid To Solid phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. We take advantage of changes between the gas,. there are six ways a substance can change between these three phases; Melting (solid → liquid) paul taylor / getty images. When the rate of condensation becomes equal to. What Is The Phase Change From Liquid To Solid.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School What Is The Phase Change From Liquid To Solid When the rate of condensation becomes equal to the rate of. to calculate the energy changes that accompany phase changes. This example shows an ice cube melting into. We take advantage of changes between the gas,. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. . What Is The Phase Change From Liquid To Solid.
From chemwiki.ucdavis.edu
Chapter 11.7 Phase Diagrams Chemwiki What Is The Phase Change From Liquid To Solid When the rate of condensation becomes equal to the rate of. This example shows an ice cube melting into. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Melting (solid → liquid) paul taylor. What Is The Phase Change From Liquid To Solid.
From www.thoughtco.com
List of Phase Changes Between States of Matter What Is The Phase Change From Liquid To Solid to calculate the energy changes that accompany phase changes. This example shows an ice cube melting into. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. When the rate of condensation becomes equal. What Is The Phase Change From Liquid To Solid.
From www.thoughtco.com
List of Phase Changes Between States of Matter What Is The Phase Change From Liquid To Solid This example shows an ice cube melting into. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. When the rate of condensation becomes equal to the rate of. to calculate the energy changes that accompany phase changes. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are. What Is The Phase Change From Liquid To Solid.
From www.radixtree.com
Physics Matter Online Education System What Is The Phase Change From Liquid To Solid This example shows an ice cube melting into. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. the change from the gas phase to the liquid is called condensation. phase changes are physical changes that take place when matter changes energy states, but chemical bonds. What Is The Phase Change From Liquid To Solid.
From www.collegesidekick.com
Solid to Gas Phase Transition Introduction to Chemistry What Is The Phase Change From Liquid To Solid to calculate the energy changes that accompany phase changes. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. When the rate of condensation becomes equal to the rate of. Melting (solid → liquid) paul taylor / getty images. This example shows an ice cube melting into.. What Is The Phase Change From Liquid To Solid.
From platoesg.com
GM Might Cool EV Charge Ports Like Microchips What Is The Phase Change From Liquid To Solid the change from the gas phase to the liquid is called condensation. This example shows an ice cube melting into. to calculate the energy changes that accompany phase changes. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. We take advantage of changes between the. What Is The Phase Change From Liquid To Solid.
From www.myxxgirl.com
Phase Change Transition Diagram States Matter Schema Evaporation My What Is The Phase Change From Liquid To Solid Melting (solid → liquid) paul taylor / getty images. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. This example shows an ice cube melting into. We take advantage of changes between the gas,. to calculate the energy changes that accompany phase changes. there are six ways a substance can change between these three. What Is The Phase Change From Liquid To Solid.
From www.coursehero.com
[Solved] According to this graph, which phase change is/are associated What Is The Phase Change From Liquid To Solid there are six ways a substance can change between these three phases; Melting (solid → liquid) paul taylor / getty images. When the rate of condensation becomes equal to the rate of. to calculate the energy changes that accompany phase changes. phase changes are physical changes that take place when matter changes energy states, but chemical bonds. What Is The Phase Change From Liquid To Solid.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock What Is The Phase Change From Liquid To Solid This example shows an ice cube melting into. the change from the gas phase to the liquid is called condensation. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. there are six ways a substance can change between these three phases; Melting (solid → liquid). What Is The Phase Change From Liquid To Solid.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to What Is The Phase Change From Liquid To Solid the change from the gas phase to the liquid is called condensation. Melting (solid → liquid) paul taylor / getty images. there are six ways a substance can change between these three phases; phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. We take advantage. What Is The Phase Change From Liquid To Solid.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Is The Phase Change From Liquid To Solid to calculate the energy changes that accompany phase changes. This example shows an ice cube melting into. Melting (solid → liquid) paul taylor / getty images. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. the change from the gas phase to the liquid is. What Is The Phase Change From Liquid To Solid.
From shaunmwilliams.com
Lecture 1 Presentation What Is The Phase Change From Liquid To Solid use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. We take advantage of changes between the gas,. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. to calculate the energy changes that accompany. What Is The Phase Change From Liquid To Solid.
From www.vectorstock.com
Different states of matter solid liquid gas Vector Image What Is The Phase Change From Liquid To Solid the change from the gas phase to the liquid is called condensation. there are six ways a substance can change between these three phases; This example shows an ice cube melting into. Melting (solid → liquid) paul taylor / getty images. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. use of one. What Is The Phase Change From Liquid To Solid.
From es.vecteezy.com
Cambiar el estado de la materia de sólido, líquido y gas debido a la What Is The Phase Change From Liquid To Solid When the rate of condensation becomes equal to the rate of. there are six ways a substance can change between these three phases; the change from the gas phase to the liquid is called condensation. We take advantage of changes between the gas,. use of one term or the other is normally dictated by the direction of. What Is The Phase Change From Liquid To Solid.
From fawe.org
爆売りセール開催中 phase What Is The Phase Change From Liquid To Solid the change from the gas phase to the liquid is called condensation. We take advantage of changes between the gas,. This example shows an ice cube melting into. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are. What Is The Phase Change From Liquid To Solid.
From jeopardylabs.com
6th Grade Science Jeopardy Template What Is The Phase Change From Liquid To Solid the change from the gas phase to the liquid is called condensation. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. to calculate the energy changes that accompany phase changes. We take. What Is The Phase Change From Liquid To Solid.
From quizlet.com
Physical and Chemical Changes Yr 8 Diagram Quizlet What Is The Phase Change From Liquid To Solid the change from the gas phase to the liquid is called condensation. Melting (solid → liquid) paul taylor / getty images. there are six ways a substance can change between these three phases; phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. to calculate. What Is The Phase Change From Liquid To Solid.
From www.theschoolrun.com
What are states of matter? TheSchoolRun What Is The Phase Change From Liquid To Solid This example shows an ice cube melting into. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. the change from the gas phase to the liquid is called condensation. to calculate the energy changes that accompany phase changes. use of one term or the other is normally dictated by the direction of the. What Is The Phase Change From Liquid To Solid.
From quizgecko.com
States of Matter Brainstorming Session What Is The Phase Change From Liquid To Solid We take advantage of changes between the gas,. the change from the gas phase to the liquid is called condensation. there are six ways a substance can change between these three phases; This example shows an ice cube melting into. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are. What Is The Phase Change From Liquid To Solid.
From www.youtube.com
Solidliquid phase diagrams YouTube What Is The Phase Change From Liquid To Solid This example shows an ice cube melting into. Melting (solid → liquid) paul taylor / getty images. We take advantage of changes between the gas,. When the rate of condensation becomes equal to the rate of. use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. the. What Is The Phase Change From Liquid To Solid.
From platoesg.com
Innovative Refrigeration Method Utilizes Phase Change Materials For What Is The Phase Change From Liquid To Solid there are six ways a substance can change between these three phases; We take advantage of changes between the gas,. to calculate the energy changes that accompany phase changes. Melting (solid → liquid) paul taylor / getty images. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken. What Is The Phase Change From Liquid To Solid.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) What Is The Phase Change From Liquid To Solid use of one term or the other is normally dictated by the direction of the phase transition being considered, for example,. to calculate the energy changes that accompany phase changes. This example shows an ice cube melting into. the change from the gas phase to the liquid is called condensation. phase changes are physical changes that. What Is The Phase Change From Liquid To Solid.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids What Is The Phase Change From Liquid To Solid This example shows an ice cube melting into. When the rate of condensation becomes equal to the rate of. sublimation (solid → gas) ionization (gas → plasma) deionization or recombination (plasma. to calculate the energy changes that accompany phase changes. Melting (solid → liquid) paul taylor / getty images. We take advantage of changes between the gas,. . What Is The Phase Change From Liquid To Solid.