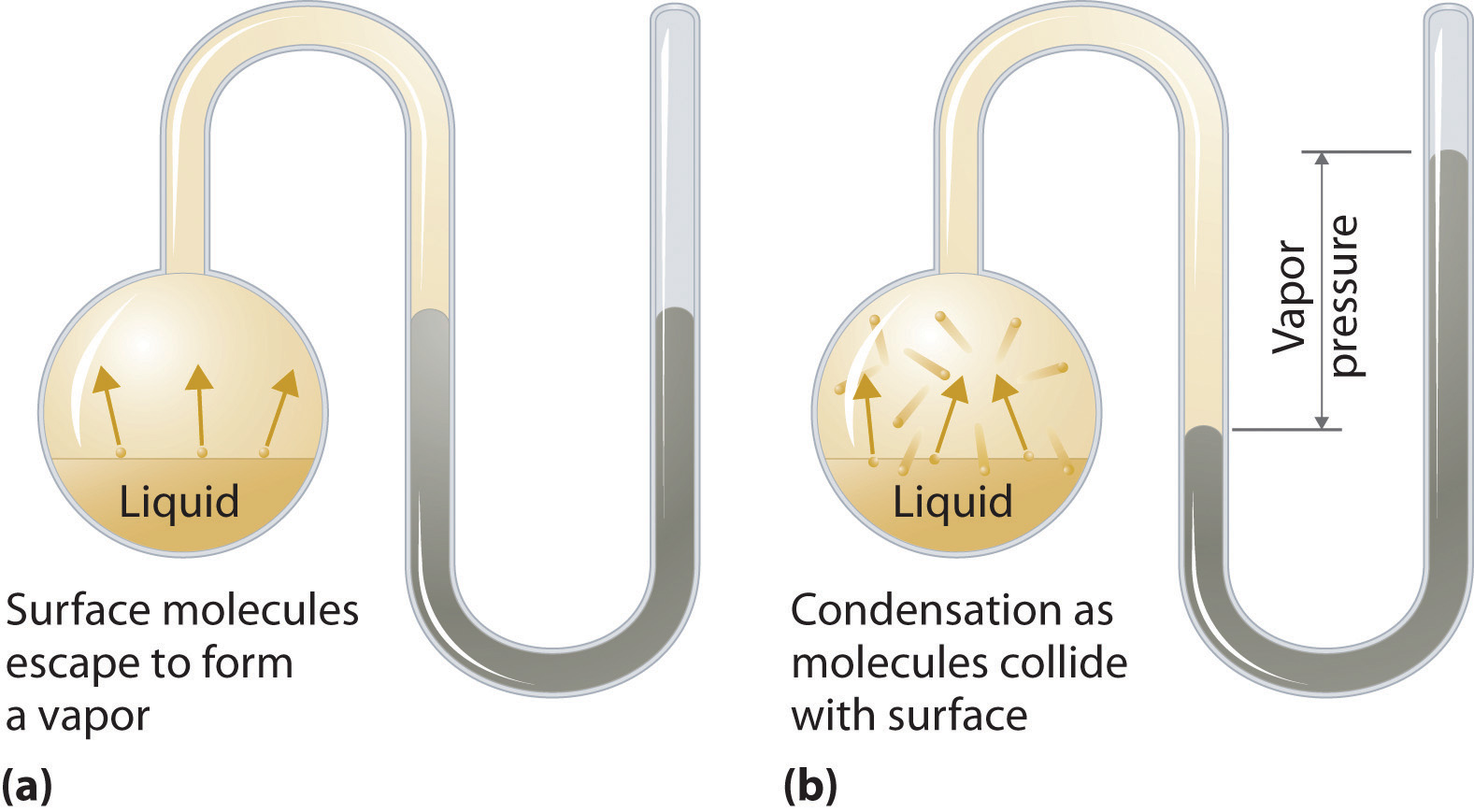
What Is Vapor Pressure For Dummies . Now, what does that definition mean? When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic.
from saylordotorg.github.io
Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Now, what does that definition mean? Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with.
Vapor Pressure
What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Now, what does that definition mean?
From slideplayer.com
Liquids and Solids Chapter ppt download What Is Vapor Pressure For Dummies When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Now, what does that definition mean? Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapor pressure or equilibrium vapor pressure is the pressure of. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure For Dummies Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Vapor Pressure Lowering PowerPoint Presentation, free download What Is Vapor Pressure For Dummies Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Now, what does that definition mean? Vapor pressure is the pressure of the. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapour pressure is a measure of the tendency. What Is Vapor Pressure For Dummies.
From chemistnotes.com
Vapor Pressure, Lowering of Vapor Pressure, Definition, Equation What Is Vapor Pressure For Dummies Now, what does that definition mean? Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at. What Is Vapor Pressure For Dummies.
From www.youtube.com
CHEMISTRY 201 Using the ClausiusClapeyron equation to solve for vapor What Is Vapor Pressure For Dummies When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Now, what does that definition mean? Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapor pressure is the pressure of the vapor over a liquid (and some solids). What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 What Is Vapor Pressure For Dummies Now, what does that definition mean? Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapour pressure is a measure of the tendency of. What Is Vapor Pressure For Dummies.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Now, what does that definition mean? Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. When. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT THE SEALED LIQUIDS PowerPoint Presentation, free download ID514680 What Is Vapor Pressure For Dummies Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Now, what does that definition mean? Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure is the pressure of the vapor over a. What Is Vapor Pressure For Dummies.
From www.wikiwand.com
Vapor pressure Wikiwand What Is Vapor Pressure For Dummies Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure is. What Is Vapor Pressure For Dummies.
From quizizz.com
Vapor Pressure Practice (Gases pg 22) questions & answers for quizzes What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with.. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure For Dummies Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape. What Is Vapor Pressure For Dummies.
From www.youtube.com
What is vapor pressure what is the vapor pressure of liquids What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. When a liquid is heated, its molecules obtain. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Now, what does that definition mean? Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapour. What Is Vapor Pressure For Dummies.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure For Dummies Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Now, what does that definition mean? When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome. What Is Vapor Pressure For Dummies.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapour pressure is a. What Is Vapor Pressure For Dummies.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Now, what does that definition mean? When. What Is Vapor Pressure For Dummies.
From pdfslide.net
(PPT) UNIT 6 SOLUTIONS AND GASES 6.6 What is vapor pressure and how do What Is Vapor Pressure For Dummies Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Now, what does that definition mean? Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. When a. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Behavior of Gases PowerPoint Presentation, free download ID3483746 What Is Vapor Pressure For Dummies When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Now, what does that definition mean? Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure. What Is Vapor Pressure For Dummies.
From slideplayer.com
Unit 9 Gases. ppt download What Is Vapor Pressure For Dummies Now, what does that definition mean? Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure For Dummies Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Now, what does that definition mean? Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapor pressure is the pressure of the vapor over a liquid (and some. What Is Vapor Pressure For Dummies.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Is Vapor Pressure For Dummies Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Now, what does that definition mean? Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them. What Is Vapor Pressure For Dummies.
From saylordotorg.github.io
Vapor Pressure What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and. What Is Vapor Pressure For Dummies.
From www.pinterest.ph
Vapor Pressure Easy Science Física What Is Vapor Pressure For Dummies When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium.. What Is Vapor Pressure For Dummies.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure For Dummies Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. When a liquid. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Aim What is the relationship between vapor pressure and boiling What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Now, what does that definition mean? Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. When. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with.. What Is Vapor Pressure For Dummies.
From studylib.net
Lesson on solutions What Is Vapor Pressure For Dummies Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Now, what does that definition mean? Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium. What Is Vapor Pressure For Dummies.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID What Is Vapor Pressure For Dummies Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Now, what does that definition mean? Vapour. What Is Vapor Pressure For Dummies.
From www.processsensing.com
Humidity Academy Theory 3 What Is Vapor Pressure For Dummies Now, what does that definition mean? Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium. What Is Vapor Pressure For Dummies.
From www.numerade.com
SOLVEDWhat is vapor pressure? What Is Vapor Pressure For Dummies Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with.. What Is Vapor Pressure For Dummies.
From jmpcoblog.com
How To Read A Pump Curve Part 2 What Is Vapor Pressure For Dummies Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Now,. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 What Is Vapor Pressure For Dummies When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in. What Is Vapor Pressure For Dummies.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Is Vapor Pressure For Dummies Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in. What Is Vapor Pressure For Dummies.
From www.slideserve.com
PPT Chapter 7 Aerosols, Sprays and Inhalations PowerPoint What Is Vapor Pressure For Dummies Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic. Now,. What Is Vapor Pressure For Dummies.