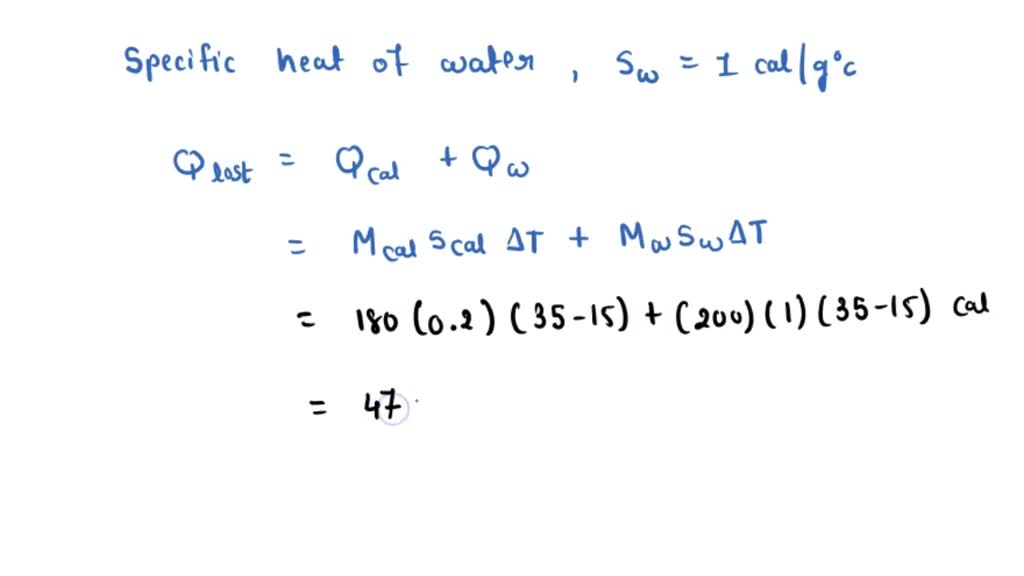Calorimetry Heat Of Fusion Of Ice Observation . A student conducts an experiment to find the latent heat of fusion for ice. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. They obtain the following table of results: Phase changes and dissolution are physical processes that absorb or release heat. The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. The collected data helps in understanding the relationship between the. The calorimeter consists of an. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. How the data relates to the purpose:
from www.numerade.com
The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. The calorimeter consists of an. How the data relates to the purpose: The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. A student conducts an experiment to find the latent heat of fusion for ice. The collected data helps in understanding the relationship between the. Phase changes and dissolution are physical processes that absorb or release heat. They obtain the following table of results: In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used.
SOLVED Calculate the heat of fusion of ice from the following data
Calorimetry Heat Of Fusion Of Ice Observation The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. A student conducts an experiment to find the latent heat of fusion for ice. The calorimeter consists of an. How the data relates to the purpose: The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. They obtain the following table of results: Phase changes and dissolution are physical processes that absorb or release heat. The collected data helps in understanding the relationship between the.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Heat Of Fusion Of Ice Observation They obtain the following table of results: The calorimeter consists of an. The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. In the first investigation, published in 1913, a calorimeter with stirred water as the. Calorimetry Heat Of Fusion Of Ice Observation.
From www.slideserve.com
PPT Heat, Temperature and Thermometers PowerPoint Presentation, free Calorimetry Heat Of Fusion Of Ice Observation In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. How the data relates to the purpose: The most straightforward method for. Calorimetry Heat Of Fusion Of Ice Observation.
From slideplayer.com
CHEM 3310 Thermodynamics. ppt download Calorimetry Heat Of Fusion Of Ice Observation How the data relates to the purpose: Phase changes and dissolution are physical processes that absorb or release heat. They obtain the following table of results: A student conducts an experiment to find the latent heat of fusion for ice. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat. Calorimetry Heat Of Fusion Of Ice Observation.
From www.studocu.com
Expt 3 Heat of Fusion rev CHEMISTRY LABORATORY Experiment 3 Calorimetry Heat Of Fusion Of Ice Observation The collected data helps in understanding the relationship between the. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. They obtain. Calorimetry Heat Of Fusion Of Ice Observation.
From sciencing.com
How to Measure Heat of Fusion of Ice Sciencing Calorimetry Heat Of Fusion Of Ice Observation The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. The calorimeter consists of an. The collected data helps in understanding the relationship between the. A student conducts an experiment to find the latent heat of. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
Pre Lab Heat of Fusion for Ice YouTube Calorimetry Heat Of Fusion Of Ice Observation Phase changes and dissolution are physical processes that absorb or release heat. The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. In the first investigation, published in 1913, a calorimeter with stirred water as the. Calorimetry Heat Of Fusion Of Ice Observation.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free Calorimetry Heat Of Fusion Of Ice Observation The calorimeter consists of an. The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. How the data relates to the purpose: They obtain the following table of results: The latent heat of melting l (j/kg),. Calorimetry Heat Of Fusion Of Ice Observation.
From www.studocu.com
Sample formal lab report Heat of fusion. Calorimetry Heat of Fusion Calorimetry Heat Of Fusion Of Ice Observation The calorimeter consists of an. The collected data helps in understanding the relationship between the. They obtain the following table of results: The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. Phase changes and dissolution are physical processes that. Calorimetry Heat Of Fusion Of Ice Observation.
From www.chegg.com
Solved Part 2. Enthalpy of fusion of ice mass of calorimeter Calorimetry Heat Of Fusion Of Ice Observation The collected data helps in understanding the relationship between the. A student conducts an experiment to find the latent heat of fusion for ice. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. In the first investigation, published in. Calorimetry Heat Of Fusion Of Ice Observation.
From cupsoguepictures.com
🎉 Discussion of theory about heat of fusion of ice. Experiment Heat of Calorimetry Heat Of Fusion Of Ice Observation The calorimeter consists of an. A student conducts an experiment to find the latent heat of fusion for ice. Phase changes and dissolution are physical processes that absorb or release heat. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. The collected data helps in understanding the relationship between the. The. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
heat of fusion of ice notes YouTube Calorimetry Heat Of Fusion Of Ice Observation They obtain the following table of results: The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. Phase changes and dissolution are physical processes that absorb or release heat. A student conducts an experiment to find. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
Calorimetry, mixing of liquids, melting of ice, latent heat of fusion Calorimetry Heat Of Fusion Of Ice Observation In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. How the data relates to the purpose: The collected data helps in understanding the relationship between the. The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
CALORIMETRY Heating Curve of ICE (3D animation) YouTube Calorimetry Heat Of Fusion Of Ice Observation A student conducts an experiment to find the latent heat of fusion for ice. Phase changes and dissolution are physical processes that absorb or release heat. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. The collected data helps. Calorimetry Heat Of Fusion Of Ice Observation.
From www.flinnsci.ca
Heat of Fusion of Ice Flinn Scientific Calorimetry Heat Of Fusion Of Ice Observation The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. A student conducts an experiment to find the latent heat of fusion for ice. In the first investigation, published in 1913, a calorimeter with stirred water. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
Latent heat of fusion of ice YouTube Calorimetry Heat Of Fusion Of Ice Observation How the data relates to the purpose: The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. They obtain the following table of results: A student conducts an experiment to find the latent heat of fusion. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
Calorimetry L8 Specific Latent Heat of Fusion of Ice ICSE Class 10 Calorimetry Heat Of Fusion Of Ice Observation In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. They obtain the following table of results: How the data relates to the purpose: The collected data helps in understanding the relationship between the. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
QUANTITY OF HEAT SPECIFIC LATENT HEAT OF FUSION OF ICE YouTube Calorimetry Heat Of Fusion Of Ice Observation Phase changes and dissolution are physical processes that absorb or release heat. A student conducts an experiment to find the latent heat of fusion for ice. The calorimeter consists of an. How the data relates to the purpose: In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. The collected data helps. Calorimetry Heat Of Fusion Of Ice Observation.
From drive.google.com
11. Calorimetry (Heat of Fusion of Ice).pdf Google Drive Calorimetry Heat Of Fusion Of Ice Observation They obtain the following table of results: The collected data helps in understanding the relationship between the. Phase changes and dissolution are physical processes that absorb or release heat. A student conducts an experiment to find the latent heat of fusion for ice. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was. Calorimetry Heat Of Fusion Of Ice Observation.
From studylib.net
Heat of Fusion of Ice Calorimetry Heat Of Fusion Of Ice Observation How the data relates to the purpose: The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the. Calorimetry Heat Of Fusion Of Ice Observation.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Calorimetry Heat Of Fusion Of Ice Observation A student conducts an experiment to find the latent heat of fusion for ice. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. The most straightforward method for measuring the specific latent heat l of ice is to drop. Calorimetry Heat Of Fusion Of Ice Observation.
From quizlet.com
Measurement of the specific latent heat of fusion of ice Flashcards Calorimetry Heat Of Fusion Of Ice Observation A student conducts an experiment to find the latent heat of fusion for ice. The collected data helps in understanding the relationship between the. They obtain the following table of results: Phase changes and dissolution are physical processes that absorb or release heat. How the data relates to the purpose: The latent heat of melting l (j/kg), also known as. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
Latent Heat of Fusion of Ice by an Experiment, Physics Lecture Sabaq Calorimetry Heat Of Fusion Of Ice Observation They obtain the following table of results: How the data relates to the purpose: The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. The collected data helps in understanding the relationship between the. In the first investigation, published in. Calorimetry Heat Of Fusion Of Ice Observation.
From www.scribd.com
Heat of Fusion of Ice PDF Heat Calorimetry Calorimetry Heat Of Fusion Of Ice Observation The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. How the data relates to the purpose: They obtain the following table of results: Phase changes and dissolution are physical processes that absorb or release heat. In the first investigation,. Calorimetry Heat Of Fusion Of Ice Observation.
From www.numerade.com
SOLVED Calculate the heat of fusion of ice from the following data Calorimetry Heat Of Fusion Of Ice Observation They obtain the following table of results: The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. Phase changes and dissolution are. Calorimetry Heat Of Fusion Of Ice Observation.
From www.slideserve.com
PPT Heat and Temperature PowerPoint Presentation, free download ID Calorimetry Heat Of Fusion Of Ice Observation A student conducts an experiment to find the latent heat of fusion for ice. They obtain the following table of results: Phase changes and dissolution are physical processes that absorb or release heat. How the data relates to the purpose: The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat. Calorimetry Heat Of Fusion Of Ice Observation.
From www.chemistrylearner.com
Heat (Enthalpy) of Fusion Definition, Equation, and Problems Calorimetry Heat Of Fusion Of Ice Observation The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. The collected data helps in understanding the relationship between the. A student conducts an experiment to find the latent heat of fusion for ice. Phase changes and dissolution are physical. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
Post Lab 7 Calorimetry Heat of Fusion of Ice YouTube Calorimetry Heat Of Fusion Of Ice Observation The collected data helps in understanding the relationship between the. A student conducts an experiment to find the latent heat of fusion for ice. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. The calorimeter consists of an. They obtain the following table of results: The most straightforward method for measuring. Calorimetry Heat Of Fusion Of Ice Observation.
From sienna-study-exams.blogspot.com
Calorimetry Heat Of Fusion Of Ice Lab Answers 22+ Pages Analysis in Doc Calorimetry Heat Of Fusion Of Ice Observation The collected data helps in understanding the relationship between the. They obtain the following table of results: A student conducts an experiment to find the latent heat of fusion for ice. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. How the data relates to the purpose: The most straightforward method. Calorimetry Heat Of Fusion Of Ice Observation.
From ucandkids.blogspot.com
Calorimetry Heat Of Fusion Of Ice Lab Answers 11+ Pages Explanation in Calorimetry Heat Of Fusion Of Ice Observation Phase changes and dissolution are physical processes that absorb or release heat. The calorimeter consists of an. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. The collected data helps in understanding the relationship between the. How the data relates to the purpose: A student conducts an experiment to find the. Calorimetry Heat Of Fusion Of Ice Observation.
From www.chegg.com
Solved Part 2. Enthalpy of fusion of ice mass of calorimeter Calorimetry Heat Of Fusion Of Ice Observation The collected data helps in understanding the relationship between the. How the data relates to the purpose: They obtain the following table of results: The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. The calorimeter consists of an. In. Calorimetry Heat Of Fusion Of Ice Observation.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation, free download ID Calorimetry Heat Of Fusion Of Ice Observation Phase changes and dissolution are physical processes that absorb or release heat. The calorimeter consists of an. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. The collected data helps in understanding the relationship between the. A student conducts. Calorimetry Heat Of Fusion Of Ice Observation.
From studylib.net
Heat of Fusion of Ice Calorimetry Heat Of Fusion Of Ice Observation The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. The calorimeter consists of an. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy. Calorimetry Heat Of Fusion Of Ice Observation.
From www.slideserve.com
PPT Experiments in L.C. Physics PowerPoint Presentation, free Calorimetry Heat Of Fusion Of Ice Observation They obtain the following table of results: The most straightforward method for measuring the specific latent heat l of ice is to drop a lump of ice of mass m and specific latent heat l at its melting point t 0. Phase changes and dissolution are physical processes that absorb or release heat. A student conducts an experiment to find. Calorimetry Heat Of Fusion Of Ice Observation.
From www.youtube.com
Measuring the Latent Heat of Fusion/Melting for Ice YouTube Calorimetry Heat Of Fusion Of Ice Observation The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. In the first investigation, published in 1913, a calorimeter with stirred water as the calorimetric medium was used. How the data relates to the purpose: They obtain the following table. Calorimetry Heat Of Fusion Of Ice Observation.
From www.vernier.com
Heat of Fusion for Ice > Experiment 4 from Chemistry with Vernier Calorimetry Heat Of Fusion Of Ice Observation They obtain the following table of results: How the data relates to the purpose: The calorimeter consists of an. The latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to supply to or take away from a. A student conducts an experiment to find the latent heat of. Calorimetry Heat Of Fusion Of Ice Observation.