Titration Indicators Procedure . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. An indicator is a dye added to a solution to change its. The reagent is usually placed in a burette and. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. The basic process involves adding a. A small amount of indicator is then added into the flask along with the analyte. There are many different types of indicators used in titration experiments. Fill the burette with the titrant solution of known concentration. Add a few drops of an indicator to the analyte solution. The solution is usually placed in a flask for titration. Which indicator is used depends on the chemistry of the reaction taking. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was.
from byjus.com
Which indicator is used depends on the chemistry of the reaction taking. An indicator is a dye added to a solution to change its. Add a few drops of an indicator to the analyte solution. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. A small amount of indicator is then added into the flask along with the analyte. There are many different types of indicators used in titration experiments. The basic process involves adding a. Fill the burette with the titrant solution of known concentration. The reagent is usually placed in a burette and. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was.
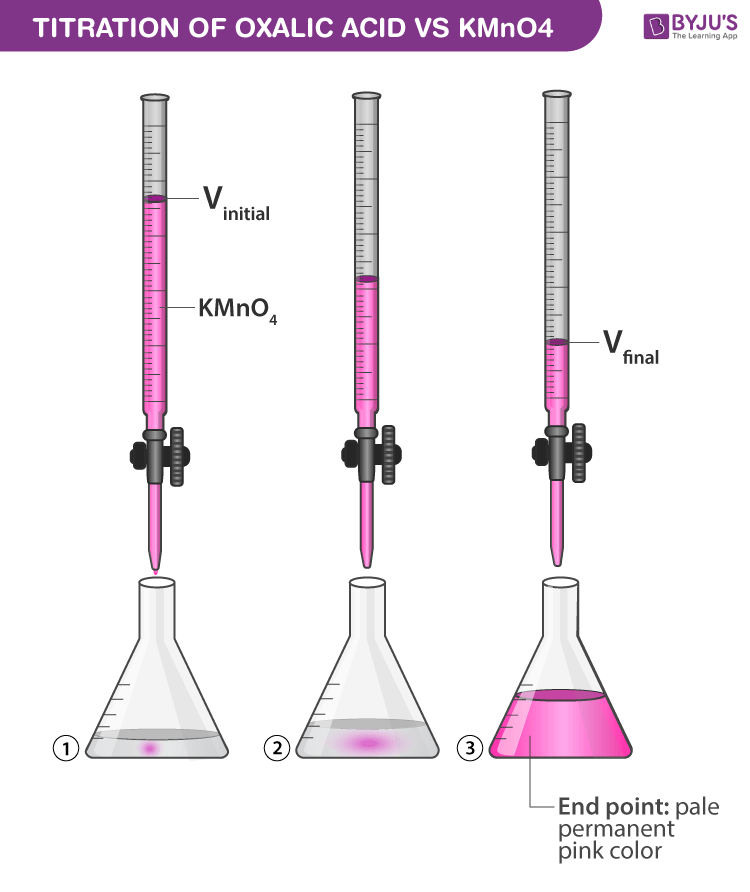
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12
Titration Indicators Procedure The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. Fill the burette with the titrant solution of known concentration. An indicator is a dye added to a solution to change its. The basic process involves adding a. The solution is usually placed in a flask for titration. A small amount of indicator is then added into the flask along with the analyte. The reagent is usually placed in a burette and. There are many different types of indicators used in titration experiments. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Which indicator is used depends on the chemistry of the reaction taking. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. Add a few drops of an indicator to the analyte solution.
From www.shutterstock.com
Acidbase Titration Setup Phenolphthalein Indicator Vector Vector có Titration Indicators Procedure The basic process involves adding a. Which indicator is used depends on the chemistry of the reaction taking. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Add a few drops of an indicator to the analyte solution. The solution is usually placed in a flask for titration. The. Titration Indicators Procedure.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Titration Indicators Procedure Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. A small amount of indicator is then added into the flask along with the analyte. The solution is usually placed in a flask for titration. The basic process involves adding a. An indicator is. Titration Indicators Procedure.
From www.hotzxgirl.com
Titration Procedure Steps Hot Sex Picture Titration Indicators Procedure A small amount of indicator is then added into the flask along with the analyte. There are many different types of indicators used in titration experiments. Which indicator is used depends on the chemistry of the reaction taking. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant. Titration Indicators Procedure.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Indicators Procedure An indicator is a dye added to a solution to change its. The reagent is usually placed in a burette and. A small amount of indicator is then added into the flask along with the analyte. Which indicator is used depends on the chemistry of the reaction taking. There are many different types of indicators used in titration experiments. Fill. Titration Indicators Procedure.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Titration Indicators Procedure The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. A small amount of indicator is then added into the flask along with the analyte. The solution is usually placed in a flask for titration. An indicator is a dye added to a solution to change its.. Titration Indicators Procedure.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Indicators Procedure Fill the burette with the titrant solution of known concentration. There are many different types of indicators used in titration experiments. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. The basic process involves adding a. The reagent is usually placed in a burette and. A. Titration Indicators Procedure.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Indicators Procedure Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. Fill the burette with the titrant solution of known concentration. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. Titration Indicators Procedure.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Indicators Procedure The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. A small amount of indicator is then added. Titration Indicators Procedure.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Titration Indicators Procedure The basic process involves adding a. Fill the burette with the titrant solution of known concentration. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Which. Titration Indicators Procedure.
From www.researchgate.net
Titration procedure to achieve optimal calcium indicator/opsin Titration Indicators Procedure Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. There are many different types of indicators used in titration experiments. The solution is usually placed in a flask for titration. An indicator is a dye added to a solution to change its. A. Titration Indicators Procedure.
From loeoavwmr.blob.core.windows.net
Selecting Indicators For AcidBase Titrations Lab at Teresa Colwell blog Titration Indicators Procedure An indicator is a dye added to a solution to change its. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Which indicator is used depends on the chemistry of the reaction taking. The volume of titrant required to neutralize the analyte could be quickly determined through the use. Titration Indicators Procedure.
From freesvg.org
Redox Titration Using Indicator Free SVG Titration Indicators Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. An indicator is a dye added to a solution to change its. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. The volume. Titration Indicators Procedure.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Indicators Procedure Add a few drops of an indicator to the analyte solution. The reagent is usually placed in a burette and. Which indicator is used depends on the chemistry of the reaction taking. There are many different types of indicators used in titration experiments. An indicator is a dye added to a solution to change its. The volume of titrant required. Titration Indicators Procedure.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Indicators Procedure Add a few drops of an indicator to the analyte solution. Which indicator is used depends on the chemistry of the reaction taking. There are many different types of indicators used in titration experiments. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a.. Titration Indicators Procedure.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 Titration Indicators Procedure The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. The reagent is usually placed in a burette and. A small amount of indicator is then added into the flask along with the analyte. Which indicator is used depends on the chemistry of the reaction taking. There. Titration Indicators Procedure.
From mungfali.com
Titration Indicators Titration Indicators Procedure Which indicator is used depends on the chemistry of the reaction taking. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are many different types of indicators used in titration experiments. The solution is usually placed in a flask for titration. The volume of titrant required to neutralize. Titration Indicators Procedure.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Indicators Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Fill the burette with the titrant solution of known concentration. The basic process involves adding a. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. Add. Titration Indicators Procedure.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Titration Indicators Procedure Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. There are many different types of indicators used in titration experiments. A small amount of indicator is then added into the flask along with the analyte. Which indicator is used depends on the chemistry. Titration Indicators Procedure.
From www.science-revision.co.uk
Titrations Titration Indicators Procedure A small amount of indicator is then added into the flask along with the analyte. An indicator is a dye added to a solution to change its. There are many different types of indicators used in titration experiments. The reagent is usually placed in a burette and. A titration is a laboratory technique used to precisely measure molar concentration of. Titration Indicators Procedure.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Indicators Procedure A small amount of indicator is then added into the flask along with the analyte. Which indicator is used depends on the chemistry of the reaction taking. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be. Titration Indicators Procedure.
From www.youtube.com
Alkalinity Buret Titration YouTube Titration Indicators Procedure Which indicator is used depends on the chemistry of the reaction taking. Fill the burette with the titrant solution of known concentration. The basic process involves adding a. There are many different types of indicators used in titration experiments. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The. Titration Indicators Procedure.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration Indicators Procedure The reagent is usually placed in a burette and. A small amount of indicator is then added into the flask along with the analyte. The solution is usually placed in a flask for titration. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. There are many. Titration Indicators Procedure.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Indicators Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. Which indicator is used depends on the chemistry of the reaction taking. An indicator. Titration Indicators Procedure.
From www.slideshare.net
Lab 3 acid base titration curves and acid_base indicators Titration Indicators Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. Fill the burette with the titrant solution of known concentration. The basic process involves adding a. Which. Titration Indicators Procedure.
From exoraquci.blob.core.windows.net
List Of Indicators Used In Titration at Jennifer Bowman blog Titration Indicators Procedure Which indicator is used depends on the chemistry of the reaction taking. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. The reagent is usually placed in a burette and. The basic process involves adding a. The solution is usually placed in a flask for titration.. Titration Indicators Procedure.
From www.youtube.com
AP Chemistry Lab 11 Proper Procedures for a Titration YouTube Titration Indicators Procedure The solution is usually placed in a flask for titration. Add a few drops of an indicator to the analyte solution. An indicator is a dye added to a solution to change its. Which indicator is used depends on the chemistry of the reaction taking. The reagent is usually placed in a burette and. Using a ph/millivoltmeter, a titration curve. Titration Indicators Procedure.
From dxonbtcto.blob.core.windows.net
Chemical Indicator AcidBase Titration at Hilda Johnston blog Titration Indicators Procedure A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The reagent is usually placed in a burette and. A small amount of indicator is then added into the flask along with the analyte. There are many different types of indicators used in titration experiments. Fill the burette with the. Titration Indicators Procedure.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Indicators Procedure A small amount of indicator is then added into the flask along with the analyte. Add a few drops of an indicator to the analyte solution. The solution is usually placed in a flask for titration. Fill the burette with the titrant solution of known concentration. There are many different types of indicators used in titration experiments. The volume of. Titration Indicators Procedure.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Titration Indicators Procedure There are many different types of indicators used in titration experiments. An indicator is a dye added to a solution to change its. The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted. Titration Indicators Procedure.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Indicators Procedure The basic process involves adding a. The reagent is usually placed in a burette and. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. Fill the burette with the titrant solution of known concentration. The solution is usually placed in a flask for. Titration Indicators Procedure.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Indicators Procedure An indicator is a dye added to a solution to change its. There are many different types of indicators used in titration experiments. The solution is usually placed in a flask for titration. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. Which. Titration Indicators Procedure.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Titration Indicators Procedure An indicator is a dye added to a solution to change its. A small amount of indicator is then added into the flask along with the analyte. There are many different types of indicators used in titration experiments. The solution is usually placed in a flask for titration. The volume of titrant required to neutralize the analyte could be quickly. Titration Indicators Procedure.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Indicators Procedure The volume of titrant required to neutralize the analyte could be quickly determined through the use of an appropriate indicator, where titrant was. The basic process involves adding a. Which indicator is used depends on the chemistry of the reaction taking. An indicator is a dye added to a solution to change its. Fill the burette with the titrant solution. Titration Indicators Procedure.
From mungfali.com
Acid Base Titration Indicator Titration Indicators Procedure A small amount of indicator is then added into the flask along with the analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The solution is usually placed in a flask for titration. Which indicator is used depends on the chemistry of the reaction taking. The reagent is. Titration Indicators Procedure.
From mungfali.com
Acid Base Titration Procedure Titration Indicators Procedure An indicator is a dye added to a solution to change its. The reagent is usually placed in a burette and. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together with a. A small amount of indicator is then added into the flask along with. Titration Indicators Procedure.