What Is The Difference Between Latent Heat And Enthalpy . Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). In this section we will develop the relationship between latent heat and enthalpy. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The latent heat of vaporization is the. Which, of course is reason that i introduced latent heats in the previous section. Enthalpy and latent heat are simply related. This energy is closely related to enthalpy. Latent heat as we have noted, you can transfer energy by heating without increasing temperature. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation.
from studylib.net
So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. Which, of course is reason that i introduced latent heats in the previous section. In this section we will develop the relationship between latent heat and enthalpy. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Enthalpy and latent heat are simply related. The latent heat of vaporization is the. Latent heat as we have noted, you can transfer energy by heating without increasing temperature. This energy is closely related to enthalpy. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid.
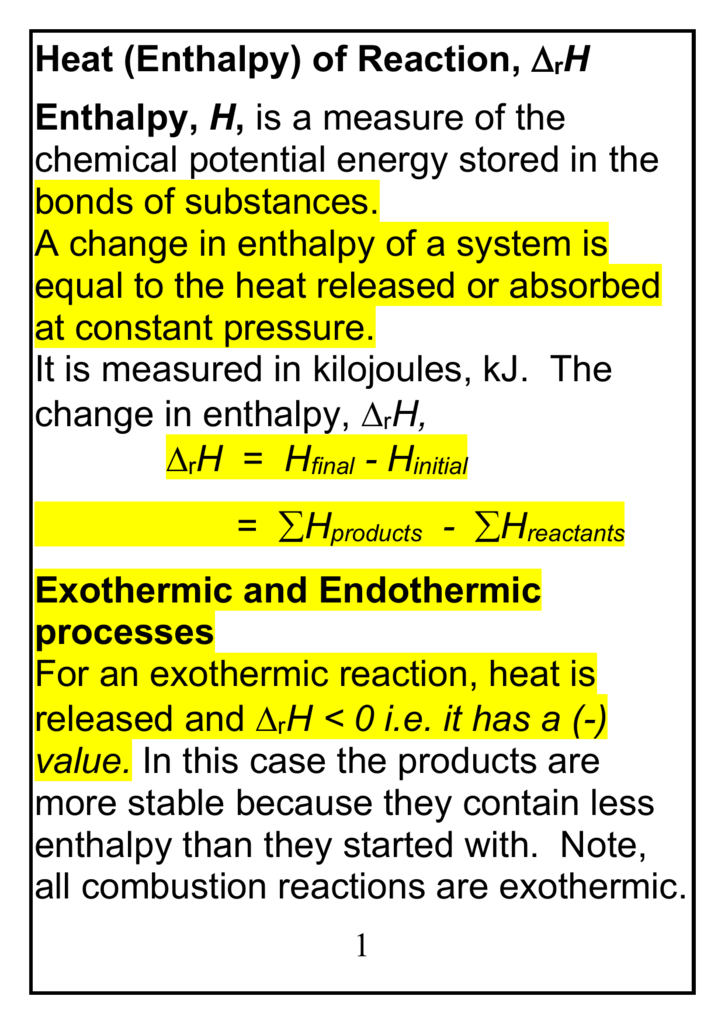
Heat (Enthalpy) of Reaction, D r H
What Is The Difference Between Latent Heat And Enthalpy Latent heat as we have noted, you can transfer energy by heating without increasing temperature. Enthalpy and latent heat are simply related. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). The latent heat of vaporization is the. So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. Latent heat as we have noted, you can transfer energy by heating without increasing temperature. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Which, of course is reason that i introduced latent heats in the previous section. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. This energy is closely related to enthalpy. In this section we will develop the relationship between latent heat and enthalpy.
From studylib.net
Heat (Enthalpy) of Reaction, D r H What Is The Difference Between Latent Heat And Enthalpy Enthalpy and latent heat are simply related. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Which, of course is reason that i introduced latent heats in the previous section. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the. What Is The Difference Between Latent Heat And Enthalpy.
From mavink.com
Types Of Latent Heat What Is The Difference Between Latent Heat And Enthalpy Which, of course is reason that i introduced latent heats in the previous section. This energy is closely related to enthalpy. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). The latent heat of vaporization is the.. What Is The Difference Between Latent Heat And Enthalpy.
From studylib.net
2.5(a) Enthalpy What Is The Difference Between Latent Heat And Enthalpy In this section we will develop the relationship between latent heat and enthalpy. Which, of course is reason that i introduced latent heats in the previous section. Enthalpy and latent heat are simply related. So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. Latent heat is. What Is The Difference Between Latent Heat And Enthalpy.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy What Is The Difference Between Latent Heat And Enthalpy The latent heat of vaporization is the. In this section we will develop the relationship between latent heat and enthalpy. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Enthalpy and latent heat are simply related. This energy is closely related to enthalpy. So, during a change of state at constant pressure the. What Is The Difference Between Latent Heat And Enthalpy.
From www.numerade.com
SOLVED Latent Heat is termed as Select one a. Entropy difference What Is The Difference Between Latent Heat And Enthalpy In this section we will develop the relationship between latent heat and enthalpy. Which, of course is reason that i introduced latent heats in the previous section. Enthalpy and latent heat are simply related. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume. What Is The Difference Between Latent Heat And Enthalpy.
From www.youtube.com
Sensible heat vs Latent HeatDifference between sensible heat and What Is The Difference Between Latent Heat And Enthalpy Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). So, during a change of state at constant pressure the increase or. What Is The Difference Between Latent Heat And Enthalpy.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download What Is The Difference Between Latent Heat And Enthalpy Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Which, of course is reason that i introduced latent heats in the previous section. Enthalpy and latent heat are simply related. This energy is closely related to enthalpy. The enthalpy is a state variable which describes the energetic state of a substance on the. What Is The Difference Between Latent Heat And Enthalpy.
From guides.co
States of Matter FD202 Fundamentals of Fire and Combustion on Guides What Is The Difference Between Latent Heat And Enthalpy The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Which, of course is reason that i introduced latent heats in the. What Is The Difference Between Latent Heat And Enthalpy.
From www.embibe.com
What is the difference between latent heat and latent heat of fusion What Is The Difference Between Latent Heat And Enthalpy In this section we will develop the relationship between latent heat and enthalpy. Which, of course is reason that i introduced latent heats in the previous section. Latent heat as we have noted, you can transfer energy by heating without increasing temperature. So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to. What Is The Difference Between Latent Heat And Enthalpy.
From www.slideserve.com
PPT Specific and Latent Heat PowerPoint Presentation ID2716332 What Is The Difference Between Latent Heat And Enthalpy This energy is closely related to enthalpy. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. Latent heat as we have noted, you can transfer energy by heating without increasing. What Is The Difference Between Latent Heat And Enthalpy.
From www.youtube.com
Changes of Heat and Specific Latent Heat YouTube What Is The Difference Between Latent Heat And Enthalpy Which, of course is reason that i introduced latent heats in the previous section. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). The latent heat of vaporization is the. Enthalpy and latent heat are simply related.. What Is The Difference Between Latent Heat And Enthalpy.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy What Is The Difference Between Latent Heat And Enthalpy Enthalpy and latent heat are simply related. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. In this section we will develop the relationship between latent heat and enthalpy. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). The latent heat of. What Is The Difference Between Latent Heat And Enthalpy.
From worksheetlistddt.z21.web.core.windows.net
Heat Energy During Phase Change What Is The Difference Between Latent Heat And Enthalpy The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). Latent heat as we have noted, you can transfer energy by heating without increasing temperature. In this section we will develop the relationship between latent heat and enthalpy.. What Is The Difference Between Latent Heat And Enthalpy.
From pediaa.com
Difference Between Enthalpy and Internal Energy What Is The Difference Between Latent Heat And Enthalpy The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. In this section we will develop the relationship between latent heat and enthalpy. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all. What Is The Difference Between Latent Heat And Enthalpy.
From www.youtube.com
Sensible heat Difference between sensible and latent heat Latent What Is The Difference Between Latent Heat And Enthalpy In this section we will develop the relationship between latent heat and enthalpy. So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. The latent heat of vaporization is the. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Latent. What Is The Difference Between Latent Heat And Enthalpy.
From www.youtube.com
"Understanding Sensible Heat vs. Latent Heat What's the Difference What Is The Difference Between Latent Heat And Enthalpy Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). This energy is closely related to enthalpy. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). Which, of course is reason. What Is The Difference Between Latent Heat And Enthalpy.
From www.askdifference.com
Enthalpy vs. Heat — What’s the Difference? What Is The Difference Between Latent Heat And Enthalpy Which, of course is reason that i introduced latent heats in the previous section. In this section we will develop the relationship between latent heat and enthalpy. So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. Enthalpy and latent heat are simply related. The latent heat. What Is The Difference Between Latent Heat And Enthalpy.
From www.savemyexams.com
Specific Heat Capacity v Specific Latent Heat AQA GCSE Physics What Is The Difference Between Latent Heat And Enthalpy The latent heat of vaporization is the. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. In this section we will develop the relationship between latent heat and enthalpy. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal. What Is The Difference Between Latent Heat And Enthalpy.
From www.diffzy.com
Specific Heat vs. Latent Heat What's The Difference (With Table) What Is The Difference Between Latent Heat And Enthalpy Latent heat as we have noted, you can transfer energy by heating without increasing temperature. This energy is closely related to enthalpy. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The enthalpy is a state variable which describes the energetic state of a substance on the basis of. What Is The Difference Between Latent Heat And Enthalpy.
From www.slideserve.com
PPT Latent heat flux PowerPoint Presentation, free download ID2715684 What Is The Difference Between Latent Heat And Enthalpy So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. This energy is closely related to enthalpy. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. In this section we will develop the relationship between latent. What Is The Difference Between Latent Heat And Enthalpy.
From examples.yourdictionary.com
Difference Between Heat and Temperature in Simple Terms YourDictionary What Is The Difference Between Latent Heat And Enthalpy In this section we will develop the relationship between latent heat and enthalpy. This energy is closely related to enthalpy. Which, of course is reason that i introduced latent heats in the previous section. So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. Latent heat as. What Is The Difference Between Latent Heat And Enthalpy.
From www.youtube.com
Difference Between Enthalpy and Internal Energy YouTube What Is The Difference Between Latent Heat And Enthalpy Latent heat as we have noted, you can transfer energy by heating without increasing temperature. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). This energy is closely related to enthalpy. The latent heat of vaporization is the. In this section we will develop the relationship between latent heat and enthalpy. The latent. What Is The Difference Between Latent Heat And Enthalpy.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk What Is The Difference Between Latent Heat And Enthalpy Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). The latent heat of vaporization is the. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The enthalpy is a state variable which describes the energetic state of a substance on the basis. What Is The Difference Between Latent Heat And Enthalpy.
From mepacademy.com
How to Calculate Sensible and Latent Heat Transfer for Air MEP Academy What Is The Difference Between Latent Heat And Enthalpy Latent heat as we have noted, you can transfer energy by heating without increasing temperature. So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and. What Is The Difference Between Latent Heat And Enthalpy.
From heatpump-reviews.com
Pressure Enthalpy Chart What Is The Difference Between Latent Heat And Enthalpy The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Enthalpy and latent heat are simply related. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). Latent. What Is The Difference Between Latent Heat And Enthalpy.
From pediaa.com
Difference Between Latent Heat and Sensible Heat Definition What Is The Difference Between Latent Heat And Enthalpy Latent heat as we have noted, you can transfer energy by heating without increasing temperature. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and. What Is The Difference Between Latent Heat And Enthalpy.
From www.researchgate.net
Idealized enthalpy content with changes in temperatures for sensible What Is The Difference Between Latent Heat And Enthalpy Enthalpy and latent heat are simply related. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. In this section we will develop the relationship between latent heat and enthalpy. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal. What Is The Difference Between Latent Heat And Enthalpy.
From www.slideserve.com
PPT Thermodynamics and Statistical Mechanics PowerPoint Presentation What Is The Difference Between Latent Heat And Enthalpy This energy is closely related to enthalpy. Enthalpy and latent heat are simply related. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume (all three variables are state variables!). Which, of course is reason that i introduced latent heats in the previous section.. What Is The Difference Between Latent Heat And Enthalpy.
From www.differencebetween.com
Difference Between Enthalpy and Heat Compare the Difference Between What Is The Difference Between Latent Heat And Enthalpy The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Which, of course is reason that i introduced latent heats in the previous section. This energy is closely related to enthalpy. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). The enthalpy is. What Is The Difference Between Latent Heat And Enthalpy.
From www.youtube.com
Enthalpy and Internal Energy YouTube What Is The Difference Between Latent Heat And Enthalpy So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Which, of course is reason that i introduced latent heats in the previous section. The enthalpy is. What Is The Difference Between Latent Heat And Enthalpy.
From ch301.cm.utexas.edu
heating curve What Is The Difference Between Latent Heat And Enthalpy Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). In this section we will develop the relationship between latent heat and enthalpy. The latent heat of vaporization is the. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure. What Is The Difference Between Latent Heat And Enthalpy.
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID6274598 What Is The Difference Between Latent Heat And Enthalpy This energy is closely related to enthalpy. In this section we will develop the relationship between latent heat and enthalpy. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Enthalpy and latent heat are simply related. Which, of course is reason that i introduced latent heats in the previous section. Latent heat as. What Is The Difference Between Latent Heat And Enthalpy.
From www.youtube.com
Change in ENTHALPY Using Specific Heat at Constant Pressure in 3 What Is The Difference Between Latent Heat And Enthalpy Which, of course is reason that i introduced latent heats in the previous section. In this section we will develop the relationship between latent heat and enthalpy. Enthalpy and latent heat are simply related. The enthalpy is a state variable which describes the energetic state of a substance on the basis of the internal energy and the pressure and volume. What Is The Difference Between Latent Heat And Enthalpy.
From www.grc.nasa.gov
Enthalpy What Is The Difference Between Latent Heat And Enthalpy So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). Enthalpy and latent heat are simply related. The latent heat of vaporization is the. The latent heat of fusion is the. What Is The Difference Between Latent Heat And Enthalpy.
From apollo.nvu.vsc.edu
Latent Heats sublimation and deposition What Is The Difference Between Latent Heat And Enthalpy So, during a change of state at constant pressure the increase or decrease of enthalpy is equal to the latent heat of transformation. In this section we will develop the relationship between latent heat and enthalpy. Which, of course is reason that i introduced latent heats in the previous section. The latent heat of vaporization is the. The latent heat. What Is The Difference Between Latent Heat And Enthalpy.