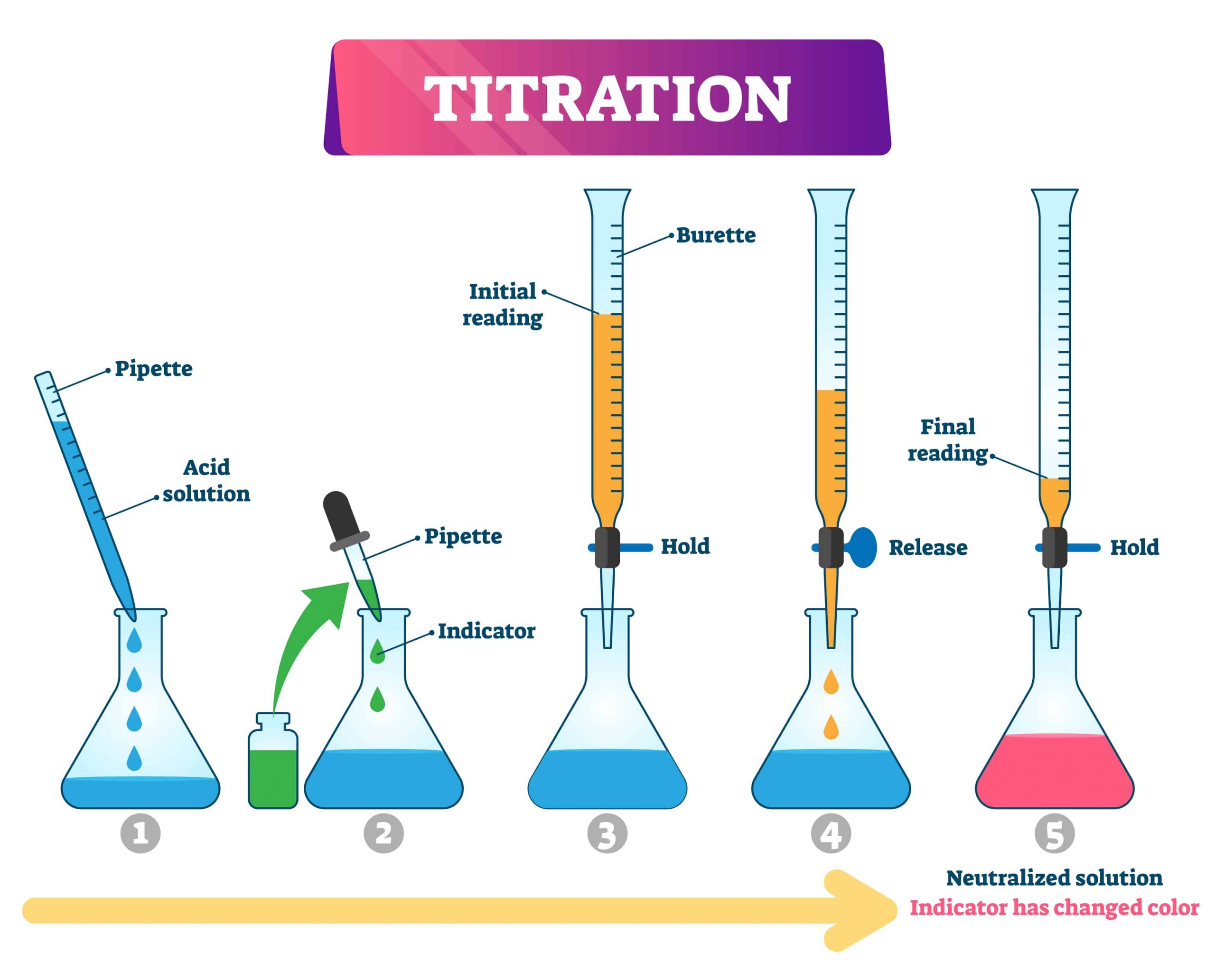
Titration Experiment Chemical Equation . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. (higher tier only) determine the concentration of one of the. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Learn the titration graph and titration equation. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine. This required practical involves using appropriate. Determine the reacting volumes of solutions of an acid and an alkali by titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. What are titrant and analyte. What is the equivalence point of a titration.
from joieldmit.blob.core.windows.net
Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. (higher tier only) determine the concentration of one of the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Determine the reacting volumes of solutions of an acid and an alkali by titration. Learn the titration graph and titration equation. This required practical involves using appropriate. What is the equivalence point of a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
How Is Titration Used In Medicine at Shelia Wiley blog
Titration Experiment Chemical Equation A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Learn the titration graph and titration equation. Determine the reacting volumes of solutions of an acid and an alkali by titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This required practical involves using appropriate. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What are titrant and analyte. What is the equivalence point of a titration. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine. (higher tier only) determine the concentration of one of the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The volumes of acids and alkali solutions that react with each other can be measured by titration using a.
From www.youtube.com
️ Titration Experiment for Board Class Complete Video to Understand Titration Experiment Chemical Equation Determine the reacting volumes of solutions of an acid and an alkali by titration. (higher tier only) determine the concentration of one of the. This required practical involves using appropriate. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. What is the equivalence point of a titration. The volumes of acids and. Titration Experiment Chemical Equation.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration Experiment Chemical Equation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce. Titration Experiment Chemical Equation.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Experiment Chemical Equation They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution. Titration Experiment Chemical Equation.
From labthink.netlify.app
What is titration and how does it work Titration Experiment Chemical Equation The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Learn the titration graph and titration equation. What is the equivalence point of a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This required. Titration Experiment Chemical Equation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Experiment Chemical Equation A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. (higher tier only) determine the concentration of one of the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. Titration Experiment Chemical Equation.
From dxobyswdg.blob.core.windows.net
Titration Means What In Chemistry at Sabrina Reyes blog Titration Experiment Chemical Equation What are titrant and analyte. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Learn the titration graph. Titration Experiment Chemical Equation.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Experiment Chemical Equation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration Experiment Chemical Equation.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Experiment Chemical Equation What are titrant and analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. This required practical involves. Titration Experiment Chemical Equation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Experiment Chemical Equation Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Learn the titration graph and titration equation. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. What is the equivalence point of a titration. Determine the reacting volumes of solutions of an acid. Titration Experiment Chemical Equation.
From www.tes.com
Acid Base Titration Theory, Titration Lab, Titration Stoichiometry Titration Experiment Chemical Equation The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Determine the reacting volumes of solutions of an acid and an alkali by titration. This required practical involves using appropriate. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another. Titration Experiment Chemical Equation.
From www.science-revision.co.uk
Titrations Titration Experiment Chemical Equation The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What are titrant and analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce. Titration Experiment Chemical Equation.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Experiment Chemical Equation A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine. (higher tier. Titration Experiment Chemical Equation.
From mungfali.com
Acid Base Titration Calculation Titration Experiment Chemical Equation Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Determine the reacting volumes of solutions of an acid and an alkali by titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A titration is a laboratory technique used to precisely measure molar concentration of an. Titration Experiment Chemical Equation.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Titration Experiment Chemical Equation (higher tier only) determine the concentration of one of the. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The volumes of acids and alkali solutions that. Titration Experiment Chemical Equation.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Experiment Chemical Equation Determine the reacting volumes of solutions of an acid and an alkali by titration. What are titrant and analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a. Titration Experiment Chemical Equation.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Titration Experiment Chemical Equation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This required practical involves using appropriate. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride. Titration Experiment Chemical Equation.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Experiment Chemical Equation Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Determine the reacting volumes of solutions of an acid and an alkali by titration. This required practical involves using appropriate. What is the equivalence point of a titration. A titration is an experiment. Titration Experiment Chemical Equation.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Experiment Chemical Equation Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order. Titration Experiment Chemical Equation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Experiment Chemical Equation Learn the titration graph and titration equation. What are titrant and analyte. Determine the reacting volumes of solutions of an acid and an alkali by titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. What is the equivalence point of a titration. (higher tier only) determine the concentration of one of the. A. Titration Experiment Chemical Equation.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Experiment Chemical Equation (higher tier only) determine the concentration of one of the. Learn the titration graph and titration equation. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Titration Experiment Chemical Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Experiment Chemical Equation This required practical involves using appropriate. Determine the reacting volumes of solutions of an acid and an alkali by titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. They then concentrate the solution and allow it. Titration Experiment Chemical Equation.
From www.youtube.com
Titration Calculations YouTube Titration Experiment Chemical Equation The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Determine the reacting volumes of solutions of an acid and an alkali by titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it. Titration Experiment Chemical Equation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Experiment Chemical Equation Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. What is the equivalence point of a titration. (higher tier only) determine the concentration of one of the. A titration is a laboratory technique. Titration Experiment Chemical Equation.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Experiment Chemical Equation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. This required practical involves using appropriate. In this experiment students neutralise sodium. Titration Experiment Chemical Equation.
From chem4three.blogspot.co.uk
CHEMISTRY 11 TITRATIONS Titration Experiment Chemical Equation Determine the reacting volumes of solutions of an acid and an alkali by titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Determination of the reacting volumes of solutions of a strong acid. Titration Experiment Chemical Equation.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Experiment Chemical Equation Learn the titration graph and titration equation. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. (higher tier only) determine the concentration of one of the. Determine the reacting volumes of solutions of. Titration Experiment Chemical Equation.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Experiment Chemical Equation They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Determine the reacting volumes of solutions of an acid. Titration Experiment Chemical Equation.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Experiment Chemical Equation A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this experiment students neutralise sodium hydroxide with. Titration Experiment Chemical Equation.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Experiment Chemical Equation A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. A titration is a laboratory technique used to precisely measure molar. Titration Experiment Chemical Equation.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment Chemical Equation The volumes of acids and alkali solutions that react with each other can be measured by titration using a. Determine the reacting volumes of solutions of an acid and an alkali by titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in. Titration Experiment Chemical Equation.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Experiment Chemical Equation Determine the reacting volumes of solutions of an acid and an alkali by titration. What is the equivalence point of a titration. What are titrant and analyte. Learn the titration graph and titration equation. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine.. Titration Experiment Chemical Equation.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Experiment Chemical Equation What is the equivalence point of a titration. (higher tier only) determine the concentration of one of the. This required practical involves using appropriate. Learn the titration graph and titration equation. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Determine the reacting volumes of solutions. Titration Experiment Chemical Equation.
From www.youtube.com
28 TITRATION 1 Experiment , Calculation HSC Chemistry YouTube Titration Experiment Chemical Equation The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. What are titrant and analyte.. Titration Experiment Chemical Equation.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Experiment Chemical Equation Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The volumes of acids and alkali solutions that react with each other can be measured by titration using a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this experiment students. Titration Experiment Chemical Equation.
From www.youtube.com
Titration Experiment Solubility Finding Equilibrium Constants Titration Experiment Chemical Equation (higher tier only) determine the concentration of one of the. Learn the titration graph and titration equation. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This required practical involves using appropriate. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They. Titration Experiment Chemical Equation.