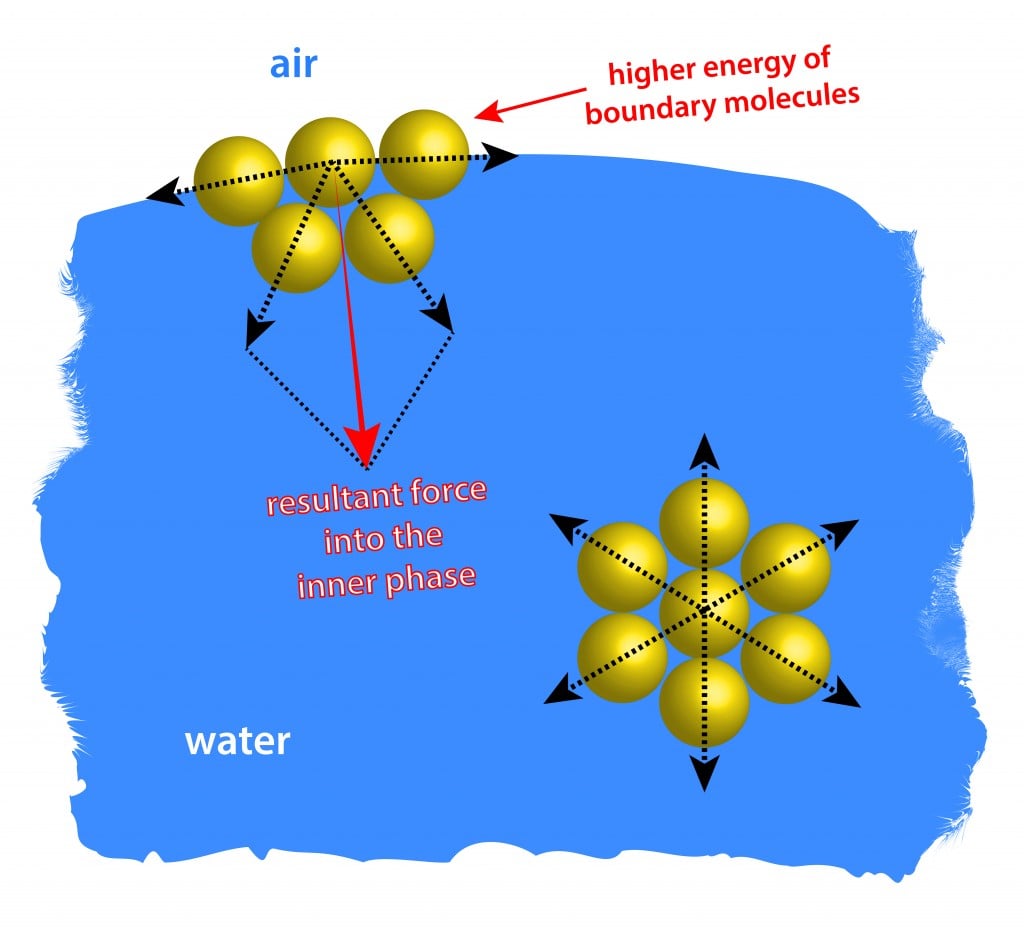
How Does Surface Tension Occur In Water . water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols,. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. This term is typically used only when the liquid surface is in contact with gas (such as the air). water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet.
from www.scienceabc.com
In comparison, organic liquids, such as benzene and alcohols,. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. This term is typically used only when the liquid surface is in contact with gas (such as the air).
Surface Tension Definition, Explanation, Examples And Significance
How Does Surface Tension Occur In Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). Water consists of one oxygen atom flanked by. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. In comparison, organic liquids, such as benzene and alcohols,. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).
From www.writework.com
Surface Tension WriteWork How Does Surface Tension Occur In Water the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: This term is typically used only when the liquid surface is in contact with gas (such as the air). In comparison, organic liquids, such as benzene and alcohols,. surface tension is a phenomenon in which the surface of a. How Does Surface Tension Occur In Water.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How Does Surface Tension Occur In Water Water consists of one oxygen atom flanked by. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but.. How Does Surface Tension Occur In Water.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant How Does Surface Tension Occur In Water the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has high surface tension, which can be explained by its polarity and hydrogen bonding. This. How Does Surface Tension Occur In Water.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts How Does Surface Tension Occur In Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water. How Does Surface Tension Occur In Water.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Occur In Water water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. In comparison, organic liquids, such as benzene and alcohols,. water has high surface tension, which can. How Does Surface Tension Occur In Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Surface Tension Occur In Water surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. In comparison, organic liquids, such as benzene and alcohols,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. This term is typically. How Does Surface Tension Occur In Water.
From www.skytechosting.com
What is Surface Tension? (With 5 examples) SkyTechosting How Does Surface Tension Occur In Water the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components. How Does Surface Tension Occur In Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Occur In Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. water has a surface tension of 0.07275 joule per square metre. How Does Surface Tension Occur In Water.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid How Does Surface Tension Occur In Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. This term is typically used only when the liquid surface is in contact with gas (such as the air). Water consists of one oxygen atom flanked by. the surface tension of a liquid results from an. How Does Surface Tension Occur In Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Occur In Water surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension of water can cause things to float which are denser than water, allowing organisms to literally. How Does Surface Tension Occur In Water.
From vectormine.com
Surface tension explanation vector illustration diagram VectorMine How Does Surface Tension Occur In Water water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water has a surface tension of 0.07275 joule per square metre. How Does Surface Tension Occur In Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Does Surface Tension Occur In Water surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. water, for example, has a very high. How Does Surface Tension Occur In Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Occur In Water Water consists of one oxygen atom flanked by. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension is a phenomenon in which the surface of a liquid, where the liquid. How Does Surface Tension Occur In Water.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Does Surface Tension Occur In Water the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. water has high surface tension, which can be explained by its polarity and hydrogen bonding.. How Does Surface Tension Occur In Water.
From exoxqkwlq.blob.core.windows.net
How Does Surface Tension Relate To Water at Ron Lowery blog How Does Surface Tension Occur In Water This term is typically used only when the liquid surface is in contact with gas (such as the air). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension in water might be good at performing tricks, such as being able to float a paper clip on. How Does Surface Tension Occur In Water.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments How Does Surface Tension Occur In Water This term is typically used only when the liquid surface is in contact with gas (such as the air). water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. . How Does Surface Tension Occur In Water.
From www.thoughtco.com
Surface Tension Definition in Chemistry How Does Surface Tension Occur In Water In comparison, organic liquids, such as benzene and alcohols,. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water has. How Does Surface Tension Occur In Water.
From ar.inspiredpencil.com
Surface Tension Water How Does Surface Tension Occur In Water Water consists of one oxygen atom flanked by. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). This term is typically used only when the liquid surface is in contact with gas (such as the air). water has high surface tension, which can be explained by its polarity and hydrogen bonding.. How Does Surface Tension Occur In Water.
From byjus.com
Determine The Surface Tension Of Water By Capillary Rise Method BYJU'S How Does Surface Tension Occur In Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such. How Does Surface Tension Occur In Water.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How Does Surface Tension Occur In Water surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. This term is typically used only when the liquid. How Does Surface Tension Occur In Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Does Surface Tension Occur In Water Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. This term is typically used only when the liquid surface is in contact with gas (such as the air). . How Does Surface Tension Occur In Water.
From www.numerade.com
At 60^∘ C the surface tension of mercury and water is 0.47 and 0.0662 N How Does Surface Tension Occur In Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact. How Does Surface Tension Occur In Water.
From www.researchgate.net
Relationship between temperature and surface tension of liquid water How Does Surface Tension Occur In Water the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. This term is typically used only when the liquid surface is in contact with gas (such. How Does Surface Tension Occur In Water.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse How Does Surface Tension Occur In Water Water consists of one oxygen atom flanked by. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact. How Does Surface Tension Occur In Water.
From aniya-has-pitts.blogspot.com
Surface Tension and Cohesion Occur in Pure Water Because Water Aniya How Does Surface Tension Occur In Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols,. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. water has high surface tension, which can be explained. How Does Surface Tension Occur In Water.
From www.biolinscientific.com
3 ways to measure surface tension How Does Surface Tension Occur In Water In comparison, organic liquids, such as benzene and alcohols,. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. This term is. How Does Surface Tension Occur In Water.
From ar.inspiredpencil.com
Surface Tension Water How Does Surface Tension Occur In Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols,. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension in water might be good at performing tricks, such as being. How Does Surface Tension Occur In Water.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… How Does Surface Tension Occur In Water Water consists of one oxygen atom flanked by. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. This term is typically used only when the liquid surface is in contact with gas (such as the air). water has high surface tension, which can be explained by its polarity. How Does Surface Tension Occur In Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii How Does Surface Tension Occur In Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water consists of one oxygen atom flanked by. . How Does Surface Tension Occur In Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download How Does Surface Tension Occur In Water In comparison, organic liquids, such as benzene and alcohols,. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). water, for example,. How Does Surface Tension Occur In Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Surface Tension Occur In Water In comparison, organic liquids, such as benzene and alcohols,. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. Water consists of one oxygen atom flanked. How Does Surface Tension Occur In Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Occur In Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension is. How Does Surface Tension Occur In Water.
From www.biolinscientific.com
Why is surface tension important? How Does Surface Tension Occur In Water the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). This term is typically used only when the liquid surface is in contact with gas (such as the air). In comparison, organic liquids,. How Does Surface Tension Occur In Water.
From www.animalia-life.club
Surface Tension Of Water On A Leaf How Does Surface Tension Occur In Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension in. How Does Surface Tension Occur In Water.
From sciencewithkids.com
Water Surface Tension Experiment Science with How Does Surface Tension Occur In Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. water has high surface tension, which can be explained by its polarity and hydrogen. How Does Surface Tension Occur In Water.