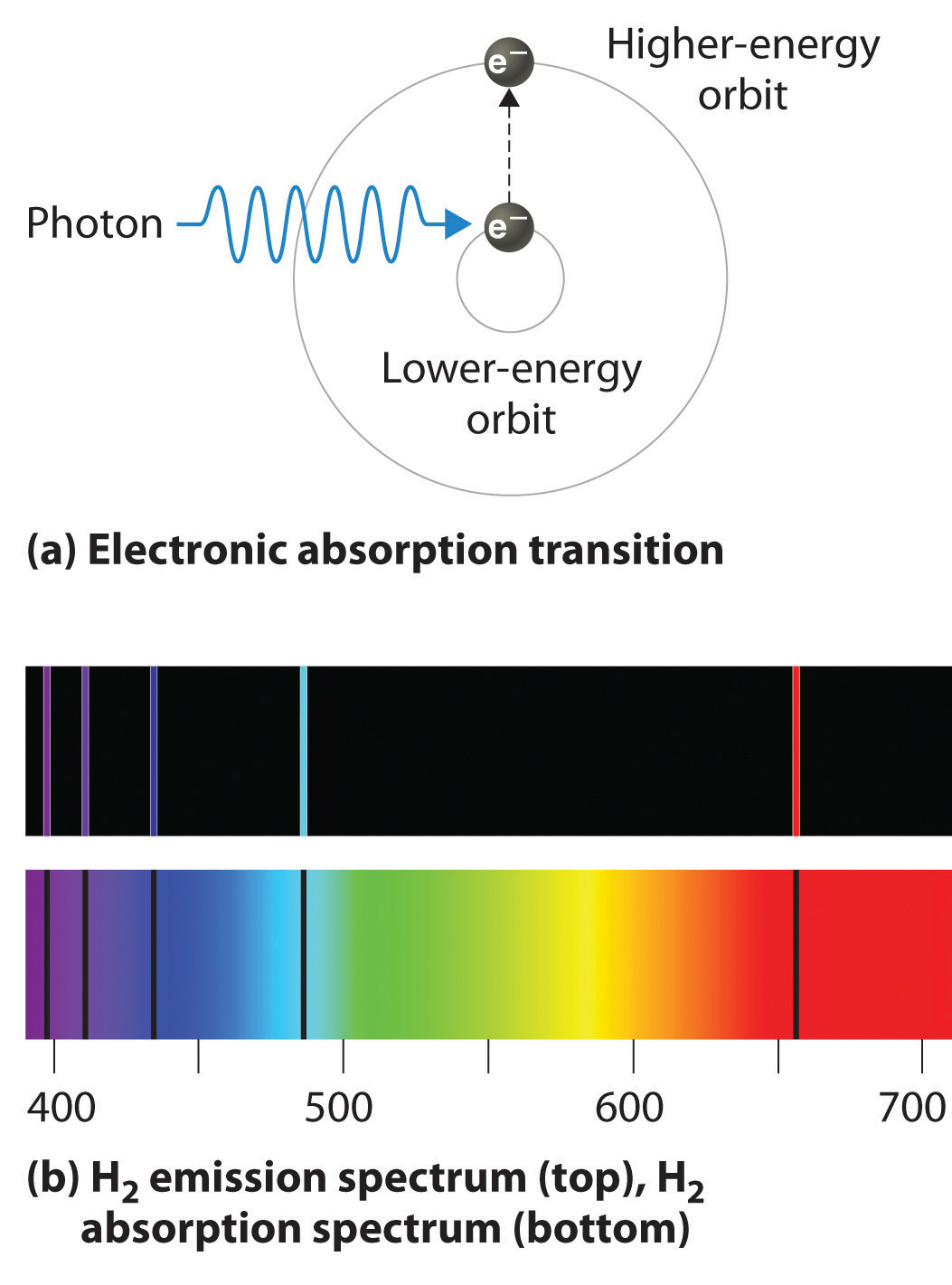
Line Spectra How Does It Work . a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. Fluorescent light bulbs and neon signs operate in this. Gases such as hydrogen are placed in a tube. explain the difference between line spectra for absorption and emission of electrons; When an atom or ion absorbs energy,. White light is passed through the tube. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. Fireworks are made up of metal salts that when heated will produce light of different colors. when heated, elements will produce line spectra. Calculate the energy associated with the electron at specified. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. steps to obtain absorption line spectrum: exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms.
from chem.libretexts.org
When an atom or ion absorbs energy,. explain the difference between line spectra for absorption and emission of electrons; a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. White light is passed through the tube. steps to obtain absorption line spectrum: atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. Gases such as hydrogen are placed in a tube. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. when heated, elements will produce line spectra. Calculate the energy associated with the electron at specified.
6.3 Line Spectra and the Bohr Model Chemistry LibreTexts
Line Spectra How Does It Work exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. Calculate the energy associated with the electron at specified. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. explain the difference between line spectra for absorption and emission of electrons; When an atom or ion absorbs energy,. Fluorescent light bulbs and neon signs operate in this. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. Gases such as hydrogen are placed in a tube. Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. White light is passed through the tube. when heated, elements will produce line spectra. Fireworks are made up of metal salts that when heated will produce light of different colors. steps to obtain absorption line spectrum:
From www.slideserve.com
PPT Honors Chemistry Unit 3 Electrons PowerPoint Presentation, free Line Spectra How Does It Work When an atom or ion absorbs energy,. Calculate the energy associated with the electron at specified. White light is passed through the tube. Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. when. Line Spectra How Does It Work.
From www.slideserve.com
PPT EMISSION SPECTRUM PowerPoint Presentation, free download ID5777174 Line Spectra How Does It Work atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. When an atom or ion absorbs energy,. explain the difference between line spectra for absorption and emission of electrons; Fluorescent light bulbs and. Line Spectra How Does It Work.
From physicscalculations.com
Line Spectra Unveiling the Secrets of Atomic Spectra Line Spectra How Does It Work a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. Gases such as hydrogen are placed in a tube. White light is passed through the tube. explain. Line Spectra How Does It Work.
From www.nagwa.com
Question Video Understanding Redshift in Spectral Lines Nagwa Line Spectra How Does It Work Fireworks are made up of metal salts that when heated will produce light of different colors. Calculate the energy associated with the electron at specified. When an atom or ion absorbs energy,. White light is passed through the tube. Gases such as hydrogen are placed in a tube. steps to obtain absorption line spectrum: a bright line spectrum. Line Spectra How Does It Work.
From spiff.rit.edu
Spectral lines and classes Line Spectra How Does It Work Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. explain the difference between line spectra for absorption and emission of electrons; White light is passed through the tube. When an atom or ion absorbs energy,. a spectral line is a spectrum in which light of only a certain wavelength is. Line Spectra How Does It Work.
From courses.lumenlearning.com
Spectroscopy in Astronomy Astronomy Line Spectra How Does It Work Calculate the energy associated with the electron at specified. steps to obtain absorption line spectrum: atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. a bright line spectrum. Line Spectra How Does It Work.
From physicsopenlab.org
Spectral Lines Broadening PhysicsOpenLab Line Spectra How Does It Work exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. steps to obtain absorption line spectrum: When an atom or ion absorbs energy,. White light is passed through the tube. when heated, elements will produce line spectra. a bright line spectrum is due to transitions of electrons. Line Spectra How Does It Work.
From www.slideserve.com
PPT Light and Atomic Spectra PowerPoint Presentation, free download Line Spectra How Does It Work steps to obtain absorption line spectrum: exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. White light is passed through the tube. Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. Calculate the energy associated with the electron at specified.. Line Spectra How Does It Work.
From fphoto.photoshelter.com
science physics waves spectrum analysis Fundamental Line Spectra How Does It Work when heated, elements will produce line spectra. Calculate the energy associated with the electron at specified. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. When an atom. Line Spectra How Does It Work.
From www.scienceabc.com
How Do We Know The Chemistry Of Things In Space? » ScienceABC Line Spectra How Does It Work Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. steps to obtain absorption line spectrum: When an atom or ion absorbs energy,. Fireworks are made up of metal salts that when heated will produce light of different colors. Fluorescent light bulbs and neon signs operate in this. a bright line. Line Spectra How Does It Work.
From jila.colorado.edu
ASTR1120002 LESSON 1 Line Spectra How Does It Work when heated, elements will produce line spectra. steps to obtain absorption line spectrum: atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. explain the difference. Line Spectra How Does It Work.
From www.artofit.org
Chapter 7 spectral lines Artofit Line Spectra How Does It Work Gases such as hydrogen are placed in a tube. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. When an atom or ion absorbs energy,. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. Fluorescent light bulbs. Line Spectra How Does It Work.
From chem.libretexts.org
6.3 Line Spectra and the Bohr Model Chemistry LibreTexts Line Spectra How Does It Work steps to obtain absorption line spectrum: Fireworks are made up of metal salts that when heated will produce light of different colors. When an atom or ion absorbs energy,. White light is passed through the tube. when heated, elements will produce line spectra. explain the difference between line spectra for absorption and emission of electrons; atoms. Line Spectra How Does It Work.
From www.slideserve.com
PPT Honors Chemistry Unit 3 Electrons PowerPoint Presentation, free Line Spectra How Does It Work Gases such as hydrogen are placed in a tube. Fireworks are made up of metal salts that when heated will produce light of different colors. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. a bright line spectrum is due to transitions of electrons between energy levels in. Line Spectra How Does It Work.
From sdsu-physics.org
Quantum Physics Line Spectra How Does It Work explain the difference between line spectra for absorption and emission of electrons; when heated, elements will produce line spectra. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. White light is passed through the tube. Fluorescent light bulbs and neon signs operate in this. a spectral line. Line Spectra How Does It Work.
From spiff.rit.edu
Spectrographs and Spectra Line Spectra How Does It Work Fluorescent light bulbs and neon signs operate in this. Fireworks are made up of metal salts that when heated will produce light of different colors. Calculate the energy associated with the electron at specified. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. White light is passed. Line Spectra How Does It Work.
From quizguarantors.z4.web.core.windows.net
Absorption Line Spectrum Line Spectra How Does It Work a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. Fluorescent light bulbs and neon signs operate in this. when heated, elements will produce line spectra. explain the. Line Spectra How Does It Work.
From www.youtube.com
Why are Characteristic Lines Produced in X Ray Spectra? YouTube Line Spectra How Does It Work when heated, elements will produce line spectra. When an atom or ion absorbs energy,. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. steps to obtain absorption line spectrum: atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. . Line Spectra How Does It Work.
From www.youtube.com
What is the Difference Between Continuous Spectrum and Line Spectrum Line Spectra How Does It Work Calculate the energy associated with the electron at specified. White light is passed through the tube. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. When an atom or ion absorbs energy,. steps to obtain absorption line spectrum: exciting a gas at low partial pressure using an electrical current,. Line Spectra How Does It Work.
From spectrometryisfun.blogspot.com
How does the spectrometer work? Atom spectroscopy Using the Line Spectra How Does It Work Fluorescent light bulbs and neon signs operate in this. a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. explain the difference between line spectra for absorption and emission of electrons; When an atom or ion absorbs energy,. White light is passed through the tube. a spectral line is a. Line Spectra How Does It Work.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Line Spectra How Does It Work a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. Gases such as hydrogen are placed in a tube. White light is passed through the tube. Calculate the energy. Line Spectra How Does It Work.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Line Spectra How Does It Work When an atom or ion absorbs energy,. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. when heated, elements will produce line spectra. steps to. Line Spectra How Does It Work.
From www.slideserve.com
PPT Electrons PowerPoint Presentation, free download ID1995426 Line Spectra How Does It Work a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. Calculate the energy associated with the electron at specified. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. Gases such as hydrogen are placed in a tube. explain the difference. Line Spectra How Does It Work.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Line Spectra How Does It Work When an atom or ion absorbs energy,. Fireworks are made up of metal salts that when heated will produce light of different colors. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. explain the difference between line spectra for absorption and emission of electrons; when heated, elements will. Line Spectra How Does It Work.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Line Spectra How Does It Work a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. when heated,. Line Spectra How Does It Work.
From www.youtube.com
Using Spectral Lines to Determine What Elements are in Stars YouTube Line Spectra How Does It Work Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. when heated, elements will produce line spectra. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. Gases such as hydrogen are placed in a tube. exciting a gas. Line Spectra How Does It Work.
From general.chemistry.msstate.edu
3.3 Atomic Line Spectra Chemistry I Line Spectra How Does It Work exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. explain the difference between line spectra for absorption and emission of electrons; when heated, elements will produce line spectra. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous.. Line Spectra How Does It Work.
From imagine.gsfc.nasa.gov
Spectra Introduction Line Spectra How Does It Work Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a. atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. Gases such. Line Spectra How Does It Work.
From www.youtube.com
S1.3.1 Line Spectra YouTube Line Spectra How Does It Work atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. Fireworks are made up of metal salts that when heated will produce light of different colors. Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. When an atom or ion absorbs energy,. Calculate. Line Spectra How Does It Work.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Line Spectra How Does It Work steps to obtain absorption line spectrum: a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. Fireworks are made up of metal salts that when heated will produce light of different colors. Gases such as hydrogen are placed in a tube. When an atom or ion absorbs energy,. White light is. Line Spectra How Does It Work.
From www.slideserve.com
PPT Chapter 3 Spectral lines in stars PowerPoint Presentation, free Line Spectra How Does It Work a bright line spectrum is due to transitions of electrons between energy levels in atoms or ions. explain the difference between line spectra for absorption and emission of electrons; Fluorescent light bulbs and neon signs operate in this. a spectral line is a spectrum in which light of only a certain wavelength is emitted or absorbed, rather. Line Spectra How Does It Work.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Line Spectra How Does It Work atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous. Fireworks are made up of metal salts that when heated will produce light of different colors. Fluorescent light bulbs and neon signs operate in this. exciting a gas at low partial pressure using an electrical current, or heating it, it. Line Spectra How Does It Work.
From mrmackenzie.co.uk
line spectra fizzics Line Spectra How Does It Work explain the difference between line spectra for absorption and emission of electrons; when heated, elements will produce line spectra. Fluorescent light bulbs and neon signs operate in this. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. steps to obtain absorption line spectrum: Line spectra, also. Line Spectra How Does It Work.
From sciencedemos.org.uk
The Spectral Lines of the Hydrogen Atom Line Spectra How Does It Work Line spectra, also known as atomic spectra, are unique patterns of light emitted or absorbed by atoms. Fluorescent light bulbs and neon signs operate in this. exciting a gas at low partial pressure using an electrical current, or heating it, it emits these lines spectra. when heated, elements will produce line spectra. When an atom or ion absorbs. Line Spectra How Does It Work.
From chem.libretexts.org
6.3 Line Spectra and the Bohr Model Chemistry LibreTexts Line Spectra How Does It Work when heated, elements will produce line spectra. White light is passed through the tube. Fireworks are made up of metal salts that when heated will produce light of different colors. Calculate the energy associated with the electron at specified. Gases such as hydrogen are placed in a tube. explain the difference between line spectra for absorption and emission. Line Spectra How Does It Work.