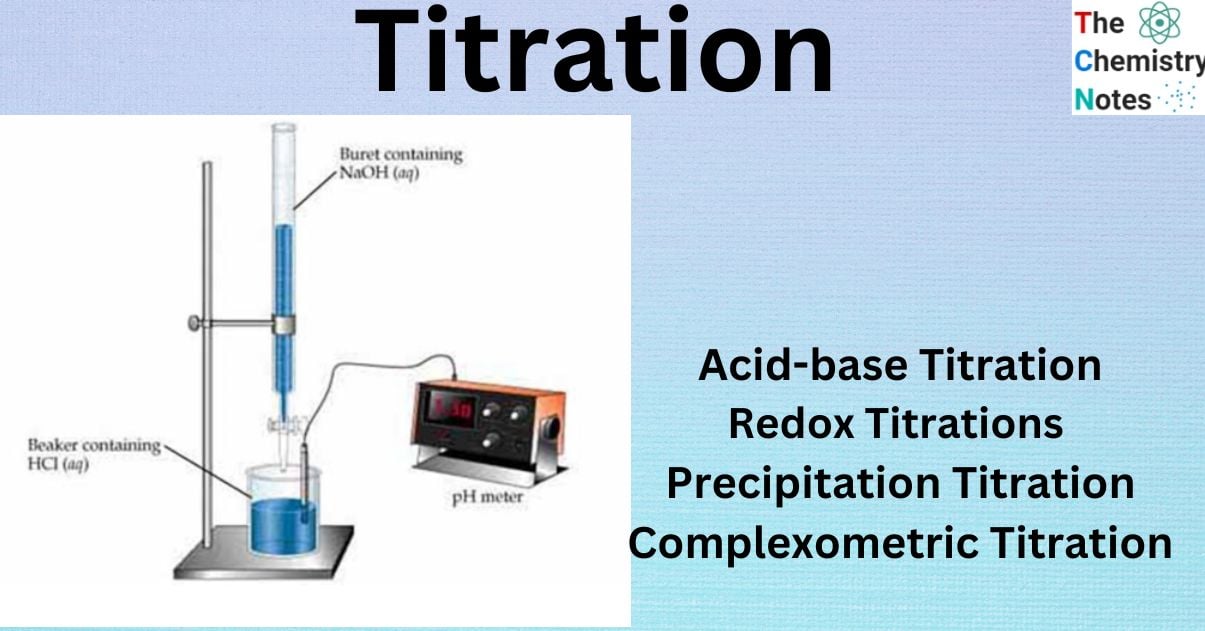
Titration Factor Definition . what is the titer factor? The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. For this, a standard solution. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. what is titration? titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titrations are used to determine the concentration of an analyte in a sample solution.
from scienceinfo.com
a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. what is the titer factor? a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titrations are used to determine the concentration of an analyte in a sample solution. what is titration? titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. For this, a standard solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent.
Titration Definition, 4 Types, Procedure
Titration Factor Definition For this, a standard solution. Titrations are used to determine the concentration of an analyte in a sample solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. what is the titer factor? Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. what is titration? method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. For this, a standard solution.
From testbook.com
Titration Formula With Definition, Process, Types, Applications Titration Factor Definition a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Factor Definition.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Factor Definition what is the titer factor? method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titrations are used to determine the concentration of an. Titration Factor Definition.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Factor Definition The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. what is the titer factor? a titration is the process of determining the quantity of one. Titration Factor Definition.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titration Factor Definition a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For this, a standard solution. Titration is an absolute method. Titration Factor Definition.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Factor Definition a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titrations are used to determine the concentration of an analyte in a sample solution. The basic process involves adding a standard solution of one reagent to a known amount of the. Titration Factor Definition.
From www.youtube.com
Normality Factor 08 Titration Class 11th & 12th YouTube Titration Factor Definition Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. For this, a standard solution. titration is the slow addition of. Titration Factor Definition.
From www.youtube.com
A Simple Guide to Titration Calculations Part 1 Concentration YouTube Titration Factor Definition Titrations are used to determine the concentration of an analyte in a sample solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. what is the titer factor? what is titration? method validation of a titration ensures. Titration Factor Definition.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Factor Definition For this, a standard solution. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. what is the titer factor? method validation of a titration ensures that the. Titration Factor Definition.
From mavink.com
Titration Diagram Labeled Titration Factor Definition For this, a standard solution. what is titration? titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titrations are used to determine the concentration of an analyte in a sample solution. a titration is a laboratory technique used to precisely measure molar concentration. Titration Factor Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Factor Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titrations are used to determine the concentration. Titration Factor Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Factor Definition a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. what is titration? a titration is a volumetric technique in which a solution of one reactant (the. Titration Factor Definition.
From fyoycwvvc.blob.core.windows.net
Titration Calculations Normality at Allen Porterfield blog Titration Factor Definition Titrations are used to determine the concentration of an analyte in a sample solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. what. Titration Factor Definition.
From gamesmartz.com
Titration Definition & Image GameSmartz Titration Factor Definition For this, a standard solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. what is titration? Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. a titration is the process of determining the quantity of. Titration Factor Definition.
From gioajmlyz.blob.core.windows.net
Titration Chemistry Research at Nicole Apple blog Titration Factor Definition method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. what is titration? For this, a standard solution. Titrations are used to determine the concentration of an analyte. Titration Factor Definition.
From exycuocgn.blob.core.windows.net
Titration Method Chemistry Gcse at Doris Baxter blog Titration Factor Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. what is the titer factor? Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. For this, a standard solution. a titration is a volumetric. Titration Factor Definition.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Factor Definition Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of. Titration Factor Definition.
From exyvlbckw.blob.core.windows.net
Karl Fischer Titration Factor Calculation at Peter Green blog Titration Factor Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. what is the titer factor? Titrations are used to determine the concentration of an analyte in a sample solution. method validation of a titration ensures that the selected titration method and parameters will provide. Titration Factor Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Factor Definition Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. For this, a standard solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. method validation of a titration ensures. Titration Factor Definition.
From worksheetcampusslewed.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Factor Definition a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. method validation of a titration ensures that the selected titration method and parameters will. Titration Factor Definition.
From themasterchemistry.com
Why Is Titration Important In Chemistry? Themasterchemistry Titration Factor Definition what is titration? Titrations are used to determine the concentration of an analyte in a sample solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and. Titration Factor Definition.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Factor Definition Titrations are used to determine the concentration of an analyte in a sample solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. The basic process involves. Titration Factor Definition.
From exymjkekq.blob.core.windows.net
Titration In Chemistry Equipment at Barbara Adamson blog Titration Factor Definition method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. For this, a standard solution. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. Titrations are used to determine the concentration of an analyte in a sample solution. . Titration Factor Definition.
From mungfali.com
Acid Base Titration Formula Titration Factor Definition Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. a titration is the process of determining the quantity of one. Titration Factor Definition.
From www.science-revision.co.uk
Titrations Titration Factor Definition Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. For this, a standard solution. method validation of a titration ensures that the selected titration method and. Titration Factor Definition.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Factor Definition a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. what is titration? method validation of a titration ensures that the selected titration method and parameters will. Titration Factor Definition.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Factor Definition The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titrations are used to determine the concentration of. Titration Factor Definition.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Titration Factor Definition a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. For this, a standard solution. titration is the slow addition of one solution of a known concentration (called a. Titration Factor Definition.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Factor Definition method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is an absolute method (or primary method), meaning it is of utmost importance to. Titration Factor Definition.
From gioxrzofe.blob.core.windows.net
Calculation Formula For Titration at Allison Rosario blog Titration Factor Definition For this, a standard solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. what is titration? Titration is an absolute method (or. Titration Factor Definition.
From easy-schule.de
Titration Definition & Zusammenfassung Easy Schule Titration Factor Definition what is the titer factor? a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. For this, a standard solution. method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. The basic process involves adding a standard solution. Titration Factor Definition.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Factor Definition a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. a titration is a laboratory technique used to precisely measure molar. Titration Factor Definition.
From www.youtube.com
titration part1 definition YouTube Titration Factor Definition a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. Titrations are used to determine the concentration of an analyte in a. Titration Factor Definition.
From studylib.net
Titration Titration Factor Definition a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. what is the titer factor? Titration is an absolute method (or primary method), meaning it is of utmost importance to know the exact concentration. For this, a standard solution. The. Titration Factor Definition.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Factor Definition method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. For this, a standard solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process involves adding a standard solution of one. Titration Factor Definition.
From relationshipbetween.com
Difference Between Normality Factor And Titration Error Relationship Titration Factor Definition The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. what is the titer factor? For this, a standard solution. a titration is a laboratory. Titration Factor Definition.