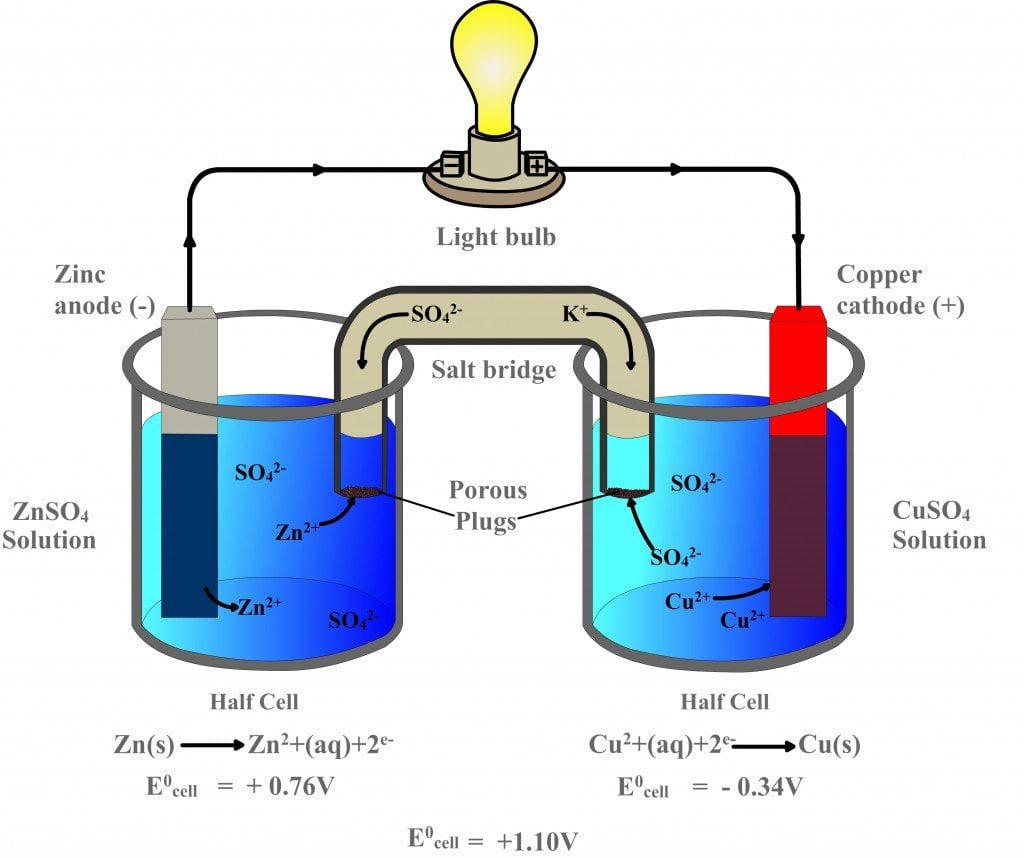
Electrochemical Galvanic Cell Difference . The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. electrochemical cells allow measurement and control of a redox reaction. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,.
from overallscience.com
a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the.
Voltaic Cell, Its Construction and Defects Overall Science
Electrochemical Galvanic Cell Difference electrochemical cells allow measurement and control of a redox reaction. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. electrochemical cells allow measurement and control of a redox reaction. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical.
From learningkaphekrs.z19.web.core.windows.net
Electrochemical Cells Gcse Chemistry Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy. Electrochemical Galvanic Cell Difference.
From 2012books.lardbucket.org
Describing Electrochemical Cells Electrochemical Galvanic Cell Difference the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. The reaction can. Electrochemical Galvanic Cell Difference.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. The reaction can. Electrochemical Galvanic Cell Difference.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Galvanic Cell Difference a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. the galvanic cell, or called voltaic cell,. Electrochemical Galvanic Cell Difference.
From hxemvfaqt.blob.core.windows.net
Example Of Irreversible Electrochemical Cell at Orville Williams blog Electrochemical Galvanic Cell Difference a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the galvanic cell, or called voltaic cell, is an electrochemical. Electrochemical Galvanic Cell Difference.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Electrochemical Galvanic Cell Difference electrochemical cells allow measurement and control of a redox reaction. The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing. Electrochemical Galvanic Cell Difference.
From hxempzkia.blob.core.windows.net
Electrochemical Cells And Cell Potentials at Gladys Johnson blog Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. A galvanic (voltaic) cell uses the energy released during a. Electrochemical Galvanic Cell Difference.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell Electrochemical Galvanic Cell Difference electrochemical cells allow measurement and control of a redox reaction. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the.. Electrochemical Galvanic Cell Difference.
From exomvxjlq.blob.core.windows.net
The Galvanic Cell Described By at Geraldo Walls blog Electrochemical Galvanic Cell Difference electrochemical cells allow measurement and control of a redox reaction. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the.. Electrochemical Galvanic Cell Difference.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Electrochemical Galvanic Cell Difference a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The reaction can be started and stopped by. Electrochemical Galvanic Cell Difference.
From hxempzkia.blob.core.windows.net
Electrochemical Cells And Cell Potentials at Gladys Johnson blog Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. electrochemical cells allow measurement and control of a redox reaction.. Electrochemical Galvanic Cell Difference.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an electrochemical cell in which spontaneous. Electrochemical Galvanic Cell Difference.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy. Electrochemical Galvanic Cell Difference.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the galvanic cell, or called voltaic cell, is an electrochemical. Electrochemical Galvanic Cell Difference.
From overallscience.com
Voltaic Cell, Its Construction and Defects Overall Science Electrochemical Galvanic Cell Difference a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction.. Electrochemical Galvanic Cell Difference.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an. Electrochemical Galvanic Cell Difference.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Electrochemical Galvanic Cell Difference a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. The reaction can be started and stopped by connecting or disconnecting the two. A galvanic (voltaic) cell uses the energy released during a spontaneous. Electrochemical Galvanic Cell Difference.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction.. Electrochemical Galvanic Cell Difference.
From enginelistmathew.z13.web.core.windows.net
Cathode In Electrochemical Cell Electrochemical Galvanic Cell Difference a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. electrochemical cells allow measurement and control. Electrochemical Galvanic Cell Difference.
From www.differencebetween.com
Difference Between Concentration Cell and Chemical Cell Compare the Electrochemical Galvanic Cell Difference a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical. Electrochemical Galvanic Cell Difference.
From worksheetfullrusskies.z21.web.core.windows.net
Diagram Of Electrochemical Cell Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell or voltaic cell, named after the. Electrochemical Galvanic Cell Difference.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrochemical Galvanic Cell Difference electrochemical cells allow measurement and control of a redox reaction. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the galvanic cell, or called voltaic cell, is an electrochemical cell that. Electrochemical Galvanic Cell Difference.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell is an. Electrochemical Galvanic Cell Difference.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The reaction can be started and stopped by. Electrochemical Galvanic Cell Difference.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Electrochemical Galvanic Cell Difference the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. electrochemical cells. Electrochemical Galvanic Cell Difference.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. electrochemical cells allow measurement and control of a redox reaction. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell. Electrochemical Galvanic Cell Difference.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Electrochemical Galvanic Cell Difference electrochemical cells allow measurement and control of a redox reaction. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell. Electrochemical Galvanic Cell Difference.
From hxemvfaqt.blob.core.windows.net
Example Of Irreversible Electrochemical Cell at Orville Williams blog Electrochemical Galvanic Cell Difference a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. electrochemical cells allow measurement and control of a redox reaction.. Electrochemical Galvanic Cell Difference.
From in.pinterest.com
three different types of electronic devices are shown in this diagram Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. The reaction can be started and stopped by connecting or disconnecting the two. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. electrochemical cells allow measurement and control of a redox. Electrochemical Galvanic Cell Difference.
From schematicploraireswsjc0.z13.web.core.windows.net
Cell Diagram For Electrochemical Cell Electrochemical Galvanic Cell Difference A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. The reaction can. Electrochemical Galvanic Cell Difference.
From guidemanualcodas.z1.web.core.windows.net
Diagram Of Voltaic Cell Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell is an electrochemical cell in which spontaneous. Electrochemical Galvanic Cell Difference.
From fixlibrarymarkbladgr.z13.web.core.windows.net
Cathode Charge In Electrolytic Cell Electrochemical Galvanic Cell Difference The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate. Electrochemical Galvanic Cell Difference.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the Electrochemical Galvanic Cell Difference electrochemical cells allow measurement and control of a redox reaction. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. The reaction can be started and stopped by connecting or disconnecting the two.. Electrochemical Galvanic Cell Difference.
From www.chemicals.co.uk
Measuring The EMF Of An Electrochemical Cell The Chemistry Blog Electrochemical Galvanic Cell Difference a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. electrochemical cells allow measurement and control of a redox reaction. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. A galvanic (voltaic) cell uses the energy released during a spontaneous redox. Electrochemical Galvanic Cell Difference.
From askfilo.com
6. Differentiate between Electrolytic cell and Galvanic cell.7. Discuss Electrochemical Galvanic Cell Difference the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. The reaction can be started and stopped by connecting or disconnecting the two. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. a galvanic cell is an. Electrochemical Galvanic Cell Difference.