How To Demonstrate Surface Tension In The Laboratory . Define the equation to solve for. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Gasoline) or solutes in the liquid (e.g. Surface tension holds the surface molecules of liquids tightly together and. Measuring surface tension with a balance beam. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. Surfactants like detergent), each solution exhibits differing surface tension properties. measure surface tension with a penny | stem activity. 7 science tricks with surface tension.
from web2.ph.utexas.edu
Surfactants like detergent), each solution exhibits differing surface tension properties. Gasoline) or solutes in the liquid (e.g. 7 science tricks with surface tension. measure surface tension with a penny | stem activity. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Measuring surface tension with a balance beam. Surface tension holds the surface molecules of liquids tightly together and. Define the equation to solve for. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given.
2A20.20 Surface Tension Lamella
How To Demonstrate Surface Tension In The Laboratory 7 science tricks with surface tension. Gasoline) or solutes in the liquid (e.g. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. 7 science tricks with surface tension. measure surface tension with a penny | stem activity. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. Measuring surface tension with a balance beam. Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension holds the surface molecules of liquids tightly together and. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Define the equation to solve for.
From www.researchgate.net
Schematic illustration of standard methods of surface tension How To Demonstrate Surface Tension In The Laboratory Surfactants like detergent), each solution exhibits differing surface tension properties. Define the equation to solve for. 7 science tricks with surface tension. Measuring surface tension with a balance beam. Surface tension holds the surface molecules of liquids tightly together and. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. surface tension. How To Demonstrate Surface Tension In The Laboratory.
From studylib.net
Surface Tension Lab (Penny Lab) How To Demonstrate Surface Tension In The Laboratory surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Surface tension holds the surface molecules of liquids tightly together and. Gasoline) or solutes in the liquid (e.g. surface tension is the energy, or work, required to. How To Demonstrate Surface Tension In The Laboratory.
From byjus.com
Explain the surface tension phenomenon with examples. How To Demonstrate Surface Tension In The Laboratory Surface tension holds the surface molecules of liquids tightly together and. Measuring surface tension with a balance beam. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Define the equation to solve for. surface tension is defined as the energy required to increase the surface area of. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
Surface tension Lab E YouTube How To Demonstrate Surface Tension In The Laboratory surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Define the equation to solve for. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. . How To Demonstrate Surface Tension In The Laboratory.
From exoytmeky.blob.core.windows.net
Determination Of Surface Tension By Ripple Method Pdf at Nicholas Fox blog How To Demonstrate Surface Tension In The Laboratory Since these intermolecular forces vary depending on the nature of the liquid (e.g. Measuring surface tension with a balance beam. Define the equation to solve for. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. to demonstrate adhesion, it is useful to place droplets of liquids on. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
Surface Tension Lab! YouTube How To Demonstrate Surface Tension In The Laboratory surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Measuring surface tension with a balance beam. Surface tension holds the surface molecules of liquids tightly together and. surface tension is the energy, or work, required to. How To Demonstrate Surface Tension In The Laboratory.
From www.instructables.com
Surface TensionThrough Expirements 4 Steps Instructables How To Demonstrate Surface Tension In The Laboratory surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. Surfactants like detergent), each solution exhibits differing surface tension properties. Measuring surface tension with a balance beam. Gasoline) or solutes in the liquid. How To Demonstrate Surface Tension In The Laboratory.
From www.dreamstime.com
Force Tensiometer To Measure Surface Tension. Laboratory Equipment How To Demonstrate Surface Tension In The Laboratory to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. 7 science tricks with surface tension. Surface tension holds the surface molecules of liquids tightly together and. surface tension is defined. How To Demonstrate Surface Tension In The Laboratory.
From web2.ph.utexas.edu
2A20.20 Surface Tension Lamella How To Demonstrate Surface Tension In The Laboratory surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Gasoline) or solutes in the liquid (e.g. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension holds the surface molecules of liquids. How To Demonstrate Surface Tension In The Laboratory.
From www.scientificamerican.com
Measure Surface Tension with a Penny Scientific American How To Demonstrate Surface Tension In The Laboratory Measuring surface tension with a balance beam. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. 7 science tricks with surface tension. Surfactants like detergent), each solution exhibits differing surface tension properties. surface tension is defined as the energy required to increase the surface area of a liquid, or the force. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
Surface tension experiment YouTube How To Demonstrate Surface Tension In The Laboratory surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Surfactants like detergent), each solution exhibits differing surface tension properties. measure surface tension with a penny | stem activity. Define the equation to solve for. to. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
School Experiment to Demonstrate Surface Tension YouTube How To Demonstrate Surface Tension In The Laboratory Define the equation to solve for. Surfactants like detergent), each solution exhibits differing surface tension properties. measure surface tension with a penny | stem activity. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension holds the surface molecules of liquids tightly together and. Measuring surface tension with a balance beam. to demonstrate. How To Demonstrate Surface Tension In The Laboratory.
From lessonschoolcarpino.z21.web.core.windows.net
How To Explain Surface Tension How To Demonstrate Surface Tension In The Laboratory Measuring surface tension with a balance beam. measure surface tension with a penny | stem activity. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Surface tension holds the surface molecules of liquids tightly together and.. How To Demonstrate Surface Tension In The Laboratory.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free How To Demonstrate Surface Tension In The Laboratory Surface tension holds the surface molecules of liquids tightly together and. 7 science tricks with surface tension. Gasoline) or solutes in the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension is defined as the energy required to increase the. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
SURFACE TENSION EXPERIMENT YouTube How To Demonstrate Surface Tension In The Laboratory 7 science tricks with surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension properties. Gasoline) or solutes in the liquid (e.g. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
Surface tension experiment part 1 YouTube How To Demonstrate Surface Tension In The Laboratory 7 science tricks with surface tension. measure surface tension with a penny | stem activity. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. Gasoline) or solutes in the liquid (e.g. Surface tension holds the surface molecules of liquids tightly together and. Measuring surface tension with a balance beam. surface. How To Demonstrate Surface Tension In The Laboratory.
From www.pinterest.com
Surface Tension Science Experiment for Kids Easy science experiments How To Demonstrate Surface Tension In The Laboratory to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. measure surface tension with a penny | stem activity. Define the equation to solve for. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
Surface Tension Lab YouTube How To Demonstrate Surface Tension In The Laboratory Surfactants like detergent), each solution exhibits differing surface tension properties. Define the equation to solve for. Measuring surface tension with a balance beam. 7 science tricks with surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. Gasoline). How To Demonstrate Surface Tension In The Laboratory.
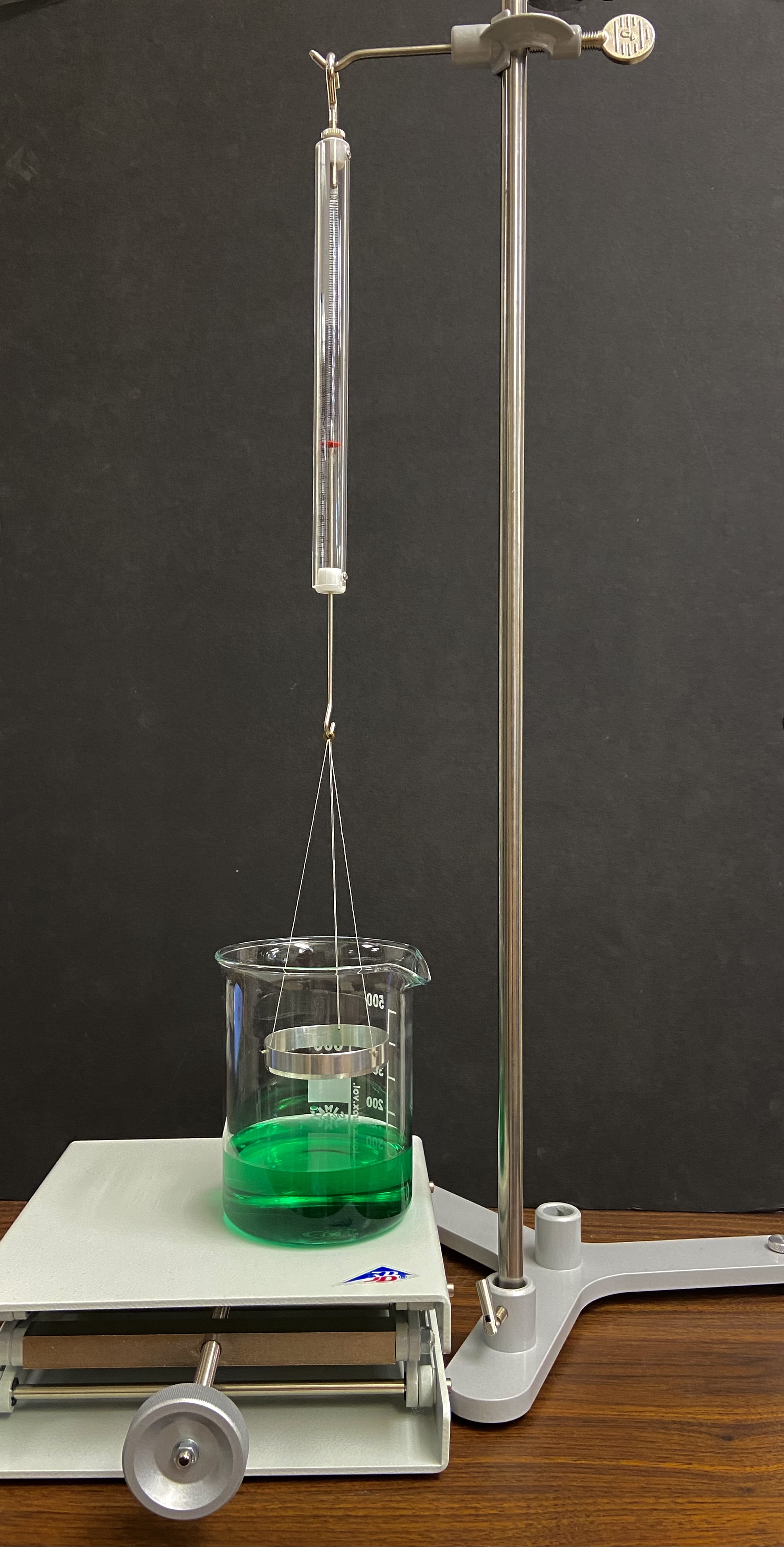
From www.3bscientific.com
Experiment Surface Tension 8000575 UE1080400 Mechanics of How To Demonstrate Surface Tension In The Laboratory surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. 7 science tricks with surface tension. Define the equation. How To Demonstrate Surface Tension In The Laboratory.
From www.alamy.com
Surface tension of water. Paper clip floats on water in the laboratory How To Demonstrate Surface Tension In The Laboratory surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension holds the surface molecules of liquids tightly together and. Measuring surface tension with a balance beam. 7 science tricks with surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. . How To Demonstrate Surface Tension In The Laboratory.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How To Demonstrate Surface Tension In The Laboratory Gasoline) or solutes in the liquid (e.g. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the. How To Demonstrate Surface Tension In The Laboratory.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How To Demonstrate Surface Tension In The Laboratory to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. 7 science tricks with surface tension. Define the equation to solve for. Surface tension holds the surface molecules of liquids tightly together. How To Demonstrate Surface Tension In The Laboratory.
From physicsexperiments.eu
Measurement of Surface Tension of Liquid — Collection of Experiments How To Demonstrate Surface Tension In The Laboratory Surface tension holds the surface molecules of liquids tightly together and. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Measuring surface tension with a balance beam. 7 science tricks with surface tension. Define the equation to solve for. Gasoline) or solutes in the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension. How To Demonstrate Surface Tension In The Laboratory.
From classful.com
Scientific Method Surface Tension Penny Lab Classful How To Demonstrate Surface Tension In The Laboratory Gasoline) or solutes in the liquid (e.g. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. measure surface tension with a penny | stem activity. Measuring surface tension with a balance beam. Surface tension holds the surface molecules of liquids tightly together and. to demonstrate adhesion,. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
How to determine Surface tension determination of liquid in lab How To Demonstrate Surface Tension In The Laboratory measure surface tension with a penny | stem activity. 7 science tricks with surface tension. Gasoline) or solutes in the liquid (e.g. Define the equation to solve for. Surfactants like detergent), each solution exhibits differing surface tension properties. surface tension is defined as the energy required to increase the surface area of a liquid, or the force. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
Surface tension experiment with paper clip YouTube How To Demonstrate Surface Tension In The Laboratory measure surface tension with a penny | stem activity. Surface tension holds the surface molecules of liquids tightly together and. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Define the equation to solve for. Surfactants. How To Demonstrate Surface Tension In The Laboratory.
From lessonlibnanometres.z13.web.core.windows.net
How To Explain Surface Tension How To Demonstrate Surface Tension In The Laboratory measure surface tension with a penny | stem activity. 7 science tricks with surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension holds the surface molecules of liquids tightly together and. Define the equation to solve for. Gasoline) or solutes in the. How To Demonstrate Surface Tension In The Laboratory.
From gosciencegirls.com
7 Surface Tension Experiments To Try With Kids How To Demonstrate Surface Tension In The Laboratory Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension properties. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e.. How To Demonstrate Surface Tension In The Laboratory.
From www.researchgate.net
Schematic illustration of standard methods of surface tension How To Demonstrate Surface Tension In The Laboratory surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surfactants like detergent), each solution exhibits differing surface tension properties. 7 science tricks with surface tension. Measuring surface tension with a balance beam. Gasoline) or solutes in the liquid (e.g. measure surface tension with a penny |. How To Demonstrate Surface Tension In The Laboratory.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How To Demonstrate Surface Tension In The Laboratory surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surfactants like detergent), each solution exhibits differing. How To Demonstrate Surface Tension In The Laboratory.
From www.alamy.com
Surface tension of water. Paper clip floats on water in the laboratory How To Demonstrate Surface Tension In The Laboratory surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. measure surface tension with a penny | stem activity. Measuring surface tension with a balance beam. to demonstrate adhesion, it is useful to place droplets of liquids on a surface (i.e. surface tension is defined as. How To Demonstrate Surface Tension In The Laboratory.
From lessonlibnanometres.z13.web.core.windows.net
Surface Tension Explained For Kids How To Demonstrate Surface Tension In The Laboratory 7 science tricks with surface tension. Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension holds the surface molecules of liquids tightly together and. Define the equation to solve for. Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension is defined as the energy required to increase the surface. How To Demonstrate Surface Tension In The Laboratory.
From www.youtube.com
Fluids Lab 1 The Theory of Surface Tension YouTube How To Demonstrate Surface Tension In The Laboratory Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Measuring surface tension with a. How To Demonstrate Surface Tension In The Laboratory.
From www.slideshare.net
Surface tension lab 2012 How To Demonstrate Surface Tension In The Laboratory Surface tension holds the surface molecules of liquids tightly together and. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension properties. Define the equation to solve for. Measuring surface tension with a balance beam. Gasoline) or solutes in the liquid (e.g. surface tension is the energy,. How To Demonstrate Surface Tension In The Laboratory.
From guidefixkattschowge.z21.web.core.windows.net
How To Explain Surface Tension How To Demonstrate Surface Tension In The Laboratory Surfactants like detergent), each solution exhibits differing surface tension properties. surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Gasoline) or solutes in the liquid (e.g. surface tension is the energy, or work, required to increase. How To Demonstrate Surface Tension In The Laboratory.