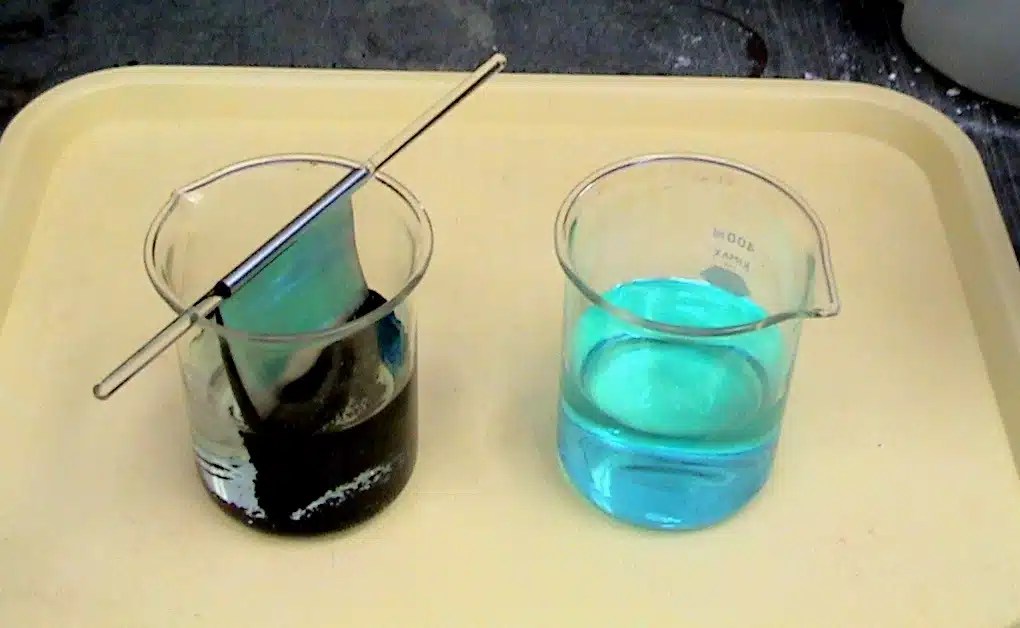
Copper Sulfate Reaction With Zinc . At the same time, the copper (ii) ions from. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Identifying the half reactions to see what got oxidized and reduced. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. What happens when you add zinc to a solution of copper sulfate? Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. Zinc + copper sulfate reaction. What happens when you add zinc to a solution of copper sulfate?
from schoolworkhelper.net
Identifying the half reactions to see what got oxidized and reduced. Zinc + copper sulfate reaction. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. What happens when you add zinc to a solution of copper sulfate? Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. At the same time, the copper (ii) ions from. What happens when you add zinc to a solution of copper sulfate?
Single Displacement Reactions Lab Explained SchoolWorkHelper
Copper Sulfate Reaction With Zinc Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zinc + copper sulfate reaction. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Identifying the half reactions to see what got oxidized and reduced. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. What happens when you add zinc to a solution of copper sulfate? When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. At the same time, the copper (ii) ions from. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. What happens when you add zinc to a solution of copper sulfate?
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Copper Sulfate Reaction With Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Identifying the half reactions to see what got oxidized and reduced. At the same time, the copper (ii) ions from. What happens when you add zinc to a solution of copper. Copper Sulfate Reaction With Zinc.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Exothermic metal displacement reactions Experiment RSC Education Copper Sulfate Reaction With Zinc If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn]. Copper Sulfate Reaction With Zinc.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Copper Sulfate Reaction With Zinc Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc + copper sulfate reaction. Identifying the. Copper Sulfate Reaction With Zinc.
From www.youtube.com
Copper (II) sulfate and zinc reactions YouTube Copper Sulfate Reaction With Zinc Identifying the half reactions to see what got oxidized and reduced. What happens when you add zinc to a solution of copper sulfate? Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. What happens when you add zinc to a solution of copper sulfate?. Copper Sulfate Reaction With Zinc.
From fphoto.photoshelter.com
science chemistry redox reaction zinc Fundamental Photographs The Copper Sulfate Reaction With Zinc Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. If left in the solution for a longer period of time, the zinc will gradually decay due. Copper Sulfate Reaction With Zinc.
From www.alamy.com
Solid metallic copper at bottom of glass beaker containing Zinc Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Identifying the half reactions to see what got. Copper Sulfate Reaction With Zinc.
From uwaterloo.ca
Zinc metal in a solution of copper(II) sulfate and sulfuric acid from Copper Sulfate Reaction With Zinc Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. Identifying the half reactions to see what got oxidized and reduced. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc + copper. Copper Sulfate Reaction With Zinc.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Copper Sulfate Reaction With Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. It is commonly known that when zinc metal is placed in a solution. Copper Sulfate Reaction With Zinc.
From fphoto.photoshelter.com
science chemistry redox reaction zinc Fundamental Photographs The Copper Sulfate Reaction With Zinc Zinc + copper sulfate reaction. Identifying the half reactions to see what got oxidized and reduced. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. At the same time, the copper (ii) ions from. What happens when you add zinc to a solution of copper sulfate? Zinc. Copper Sulfate Reaction With Zinc.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. What happens when you add zinc to a solution of copper sulfate? Zinc is a more reactive metal than copper, thus it. Copper Sulfate Reaction With Zinc.
From www.youtube.com
Iron metal + Copper(II) sulfate solution reaction Timelapse 100x YouTube Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. What happens when you add zinc to a solution of copper sulfate? When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu + znso4 is a single. Copper Sulfate Reaction With Zinc.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Copper Sulfate Reaction With Zinc It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. Zinc + copper sulfate reaction. What happens when you add zinc to a solution. Copper Sulfate Reaction With Zinc.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Copper Sulfate Reaction With Zinc Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction. Copper Sulfate Reaction With Zinc.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper Sulfate Reaction With Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. It is commonly known that when. Copper Sulfate Reaction With Zinc.
From en.ppt-online.org
Metals online presentation Copper Sulfate Reaction With Zinc It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. At the same time, the copper (ii) ions from. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as. Copper Sulfate Reaction With Zinc.
From brunofuga.adv.br
Cuso4 Zn Reaction Online Clearance brunofuga.adv.br Copper Sulfate Reaction With Zinc Identifying the half reactions to see what got oxidized and reduced. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to. Copper Sulfate Reaction With Zinc.
From www.chegg.com
Solved mical Reactions REPORT Zinc and Copper Sulfate The Copper Sulfate Reaction With Zinc It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. What happens when. Copper Sulfate Reaction With Zinc.
From www.sciencephoto.com
Zinc, Copper Sulphate Reaction, 1 of 2 Stock Image C030/7890 Copper Sulfate Reaction With Zinc Zinc + copper sulfate reaction. What happens when you add zinc to a solution of copper sulfate? At the same time, the copper (ii) ions from. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When a strip of zinc metal. Copper Sulfate Reaction With Zinc.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0148 Science Copper Sulfate Reaction With Zinc Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Identifying the half reactions to see what got oxidized and reduced. What happens when you add zinc to a solution of copper sulfate? At the same time, the copper (ii) ions from. Zinc + copper. Copper Sulfate Reaction With Zinc.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Copper Sulfate Reaction With Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. If left in the solution for a longer period of time, the zinc. Copper Sulfate Reaction With Zinc.
From brainly.in
Vidisha placed a zinc plate in copper sulphate solution as shown in the Copper Sulfate Reaction With Zinc It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Identifying the half reactions to see what got oxidized and reduced. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. When a strip. Copper Sulfate Reaction With Zinc.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Copper Sulfate Reaction With Zinc Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. What happens when you add zinc to a solution of copper sulfate? It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental. Copper Sulfate Reaction With Zinc.
From www.slideshare.net
Replacement reactions Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. What happens when you add zinc to a solution of copper sulfate? Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. If left in the solution for a longer period of time, the zinc will. Copper Sulfate Reaction With Zinc.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Copper Sulfate Reaction With Zinc What happens when you add zinc to a solution of copper sulfate? Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. What happens when you add zinc to a solution of copper sulfate? Zinc + copper sulfate reaction. At the same time, the copper. Copper Sulfate Reaction With Zinc.
From spark.adobe.com
Zinc and Copper Chloride Reaction Copper Sulfate Reaction With Zinc Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. At the same time, the copper (ii) ions from. Identifying the half reactions to. Copper Sulfate Reaction With Zinc.
From coggle.it
Rate of Chemical Reactions (Zinc + Copper Sulfate Reaction (Observable… Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. What happens when you add zinc to a solution of copper sulfate? What happens when you add zinc to a solution of copper sulfate? It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto. Copper Sulfate Reaction With Zinc.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Copper Sulfate Reaction With Zinc Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Identifying the half reactions to see what got oxidized and reduced. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper. Copper Sulfate Reaction With Zinc.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. Identifying the half reactions to see what got oxidized and reduced. What happens when you add zinc to a solution of copper sulfate? It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the.. Copper Sulfate Reaction With Zinc.
From www.youtube.com
Copper Sulfate + Zinc YouTube Copper Sulfate Reaction With Zinc It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc + copper sulfate reaction. Identifying the half reactions to see what got oxidized and reduced. What happens when you add zinc to a solution of copper sulfate? What happens when you. Copper Sulfate Reaction With Zinc.
From www.toppr.com
What happens when a piece of silver metal is added to the copper Copper Sulfate Reaction With Zinc If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. At the same time, the copper (ii) ions from. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zinc is a more. Copper Sulfate Reaction With Zinc.
From www.science-revision.co.uk
Displacement reactions Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. What. Copper Sulfate Reaction With Zinc.
From www.sciencephoto.com
Zinc, Copper Sulphate Reaction, 2 of 2 Stock Image C030/7891 Copper Sulfate Reaction With Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. What happens when you add zinc to a solution of copper sulfate? Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. Zinc. Copper Sulfate Reaction With Zinc.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper Sulfate Reaction With Zinc Zinc + copper sulfate reaction. What happens when you add zinc to a solution of copper sulfate? It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When a strip of zinc metal is placed into a blue solution of copper (ii). Copper Sulfate Reaction With Zinc.
From www.youtube.com
Zinc and Copper Sulphate reaction (Displacement Reaction) Zn and CuSO4 Copper Sulfate Reaction With Zinc At the same time, the copper (ii) ions from. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Identifying the half reactions to see what got oxidized and reduced. What happens when you add zinc to a solution of copper sulfate? Zinc is a. Copper Sulfate Reaction With Zinc.
From www.sciencephoto.com
Nickel Reacting With Copper Sulfate Stock Image C028/0162 Science Copper Sulfate Reaction With Zinc If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. When a strip of zinc metal is placed into a blue solution of. Copper Sulfate Reaction With Zinc.