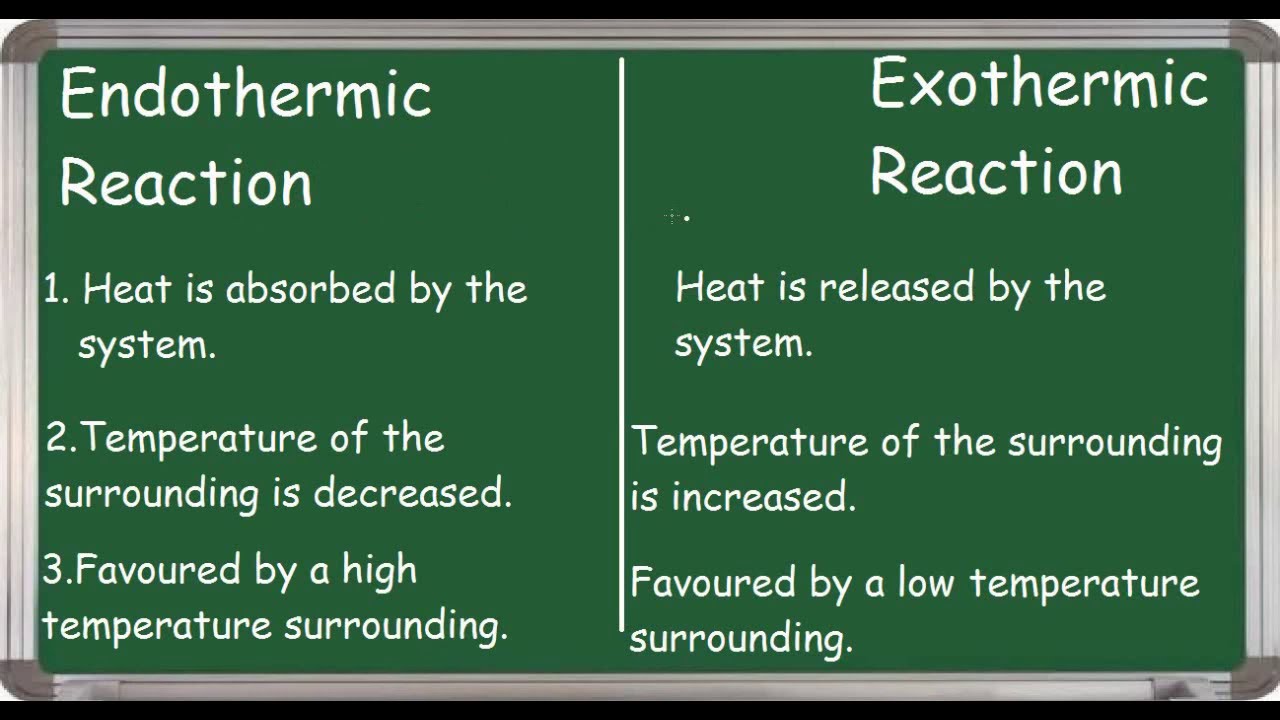
Is Water Boiling Endothermic Or Exothermic . Factors such as atmospheric pressure,. This means it absorbs heat from its surroundings. The process of boiling water is endothermic. Boiling water is an endothermic process. This is mostly because heat must be applied in order for water to boil. Because you are adding heat/energy, the reaction is endothermic. Boiling water is an endothermic process that absorbs heat from the surroundings. Therefore, boiling water is an endothermic process. The applied heat helps break the intermolecular bonds between the water. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs thermal. The absorption of heat during boiling results in a cooling effect. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. This is because energy is required to break the hydrogen.
from klajlbzfp.blob.core.windows.net
This is mostly because heat must be applied in order for water to boil. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs thermal. Boiling water is an endothermic process that absorbs heat from the surroundings. Boiling water is an endothermic process. This is because energy is required to break the hydrogen. Because you are adding heat/energy, the reaction is endothermic. This means it absorbs heat from its surroundings. The applied heat helps break the intermolecular bonds between the water. Therefore, boiling water is an endothermic process.
Endothermic Solution Examples at Jeffrey Pleasants blog
Is Water Boiling Endothermic Or Exothermic Boiling water is an endothermic process that absorbs heat from the surroundings. This is because energy is required to break the hydrogen. This is mostly because heat must be applied in order for water to boil. Boiling water is an endothermic process that absorbs heat from the surroundings. Boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The applied heat helps break the intermolecular bonds between the water. Factors such as atmospheric pressure,. The absorption of heat during boiling results in a cooling effect. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Because you are adding heat/energy, the reaction is endothermic. An endothermic reaction is a chemical reaction that absorbs thermal. The process of boiling water is endothermic. Therefore, boiling water is an endothermic process. This means it absorbs heat from its surroundings.
From www.pinterest.co.uk
Exothermic reactions release energy. Endothermic reactions consume Is Water Boiling Endothermic Or Exothermic Therefore, boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. This means it absorbs heat from its surroundings. This is because energy is required to break the hydrogen. The applied heat helps break the intermolecular bonds between the water. Boiling water is an endothermic process. An endothermic reaction is. Is Water Boiling Endothermic Or Exothermic.
From slideplayer.com
LO 3.11 The student is able to interpret observations regarding Is Water Boiling Endothermic Or Exothermic In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The absorption of heat during boiling results in a cooling effect. Boiling water is an endothermic process. Boiling water is an endothermic process that absorbs heat from. Is Water Boiling Endothermic Or Exothermic.
From www.youtube.com
Is boiling water endothermic or exothermic with respect to water? YouTube Is Water Boiling Endothermic Or Exothermic Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Boiling water is an endothermic process. Boiling water is an endothermic process that absorbs heat from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal. This means it absorbs heat from its surroundings. In thermodynamics, an endothermic process is one that absorbs heat. Is Water Boiling Endothermic Or Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Is Water Boiling Endothermic Or Exothermic Factors such as atmospheric pressure,. Boiling water is an endothermic process that absorbs heat from the surroundings. This is mostly because heat must be applied in order for water to boil. Boiling water is an endothermic process. The applied heat helps break the intermolecular bonds between the water. An endothermic reaction is a chemical reaction that absorbs thermal. Examples of. Is Water Boiling Endothermic Or Exothermic.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Is Water Boiling Endothermic Or Exothermic Factors such as atmospheric pressure,. This means it absorbs heat from its surroundings. This is mostly because heat must be applied in order for water to boil. Boiling water is an endothermic process. This is because energy is required to break the hydrogen. Therefore, boiling water is an endothermic process. The absorption of heat during boiling results in a cooling. Is Water Boiling Endothermic Or Exothermic.
From exobyexop.blob.core.windows.net
Is Butane Gas Burning In A Lighter Exothermic Or Endothermic at Chanel Is Water Boiling Endothermic Or Exothermic Boiling water is an endothermic process that absorbs heat from the surroundings. The applied heat helps break the intermolecular bonds between the water. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. This is mostly because heat must be applied in order for water to boil. Therefore, boiling water is an endothermic process. This means. Is Water Boiling Endothermic Or Exothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Is Water Boiling Endothermic Or Exothermic The applied heat helps break the intermolecular bonds between the water. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. An endothermic reaction is a chemical reaction that absorbs thermal. Boiling water is an endothermic process that absorbs heat from the surroundings. This is because energy is required to break. Is Water Boiling Endothermic Or Exothermic.
From slideplayer.com
Chemistry Unit 2 Matter and Energy. ppt download Is Water Boiling Endothermic Or Exothermic Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Because you are adding heat/energy, the reaction is endothermic. Therefore, boiling water is an endothermic process. An endothermic reaction is a chemical reaction that absorbs thermal. Factors such as atmospheric pressure,. This is because energy is required to break the hydrogen. The process of boiling water. Is Water Boiling Endothermic Or Exothermic.
From sciencenotes.org
Exothermic Reactions Definition and Examples Is Water Boiling Endothermic Or Exothermic Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The absorption of heat during boiling results in a cooling effect. This is because energy is required to break the hydrogen. This is mostly because heat must be applied in order for water to boil. The applied heat helps break the intermolecular bonds between the water.. Is Water Boiling Endothermic Or Exothermic.
From slideplayer.com
Chapter 10 Energy. ppt download Is Water Boiling Endothermic Or Exothermic Because you are adding heat/energy, the reaction is endothermic. Factors such as atmospheric pressure,. The applied heat helps break the intermolecular bonds between the water. The absorption of heat during boiling results in a cooling effect. Therefore, boiling water is an endothermic process. This is mostly because heat must be applied in order for water to boil. Examples of endothermic. Is Water Boiling Endothermic Or Exothermic.
From worksheetfullcrandall.z21.web.core.windows.net
Heat And Cooling Curve Is Water Boiling Endothermic Or Exothermic This is mostly because heat must be applied in order for water to boil. The applied heat helps break the intermolecular bonds between the water. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Because you are adding heat/energy, the reaction is endothermic. Factors such as atmospheric pressure,. Examples of. Is Water Boiling Endothermic Or Exothermic.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Is Water Boiling Endothermic Or Exothermic Boiling water is an endothermic process that absorbs heat from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal. The applied heat helps break the intermolecular bonds between the water. The absorption of heat during boiling results in a cooling effect. Therefore, boiling water is an endothermic process. This is because energy is required to break the. Is Water Boiling Endothermic Or Exothermic.
From www.ck12.org
Energy Change in Reactions CK12 Foundation Is Water Boiling Endothermic Or Exothermic This means it absorbs heat from its surroundings. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Therefore, boiling water is an endothermic process. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. The process of boiling water is endothermic. This is because energy is. Is Water Boiling Endothermic Or Exothermic.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Is Water Boiling Endothermic Or Exothermic Therefore, boiling water is an endothermic process. The process of boiling water is endothermic. An endothermic reaction is a chemical reaction that absorbs thermal. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Factors such as atmospheric pressure,. The applied heat helps break the intermolecular bonds between the water. This. Is Water Boiling Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic Reaction Examples Is Water Boiling Endothermic Or Exothermic Boiling water is an endothermic process that absorbs heat from the surroundings. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The applied heat helps break the intermolecular bonds between the water. This is mostly because heat must be applied in order for water to boil. The absorption of heat during boiling results in a. Is Water Boiling Endothermic Or Exothermic.
From vhmsscience8.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Is Water Boiling Endothermic Or Exothermic The process of boiling water is endothermic. This is mostly because heat must be applied in order for water to boil. The absorption of heat during boiling results in a cooling effect. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Because you are adding heat/energy, the reaction is endothermic. In thermodynamics, an endothermic process. Is Water Boiling Endothermic Or Exothermic.
From exokxtgqu.blob.core.windows.net
Endothermic Reaction Meaning In Urdu at Kerry Hale blog Is Water Boiling Endothermic Or Exothermic The applied heat helps break the intermolecular bonds between the water. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. This is mostly because heat must be applied in order for water to boil. The process of boiling water is endothermic. The absorption of heat during boiling results in a. Is Water Boiling Endothermic Or Exothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Is Water Boiling Endothermic Or Exothermic The process of boiling water is endothermic. Because you are adding heat/energy, the reaction is endothermic. An endothermic reaction is a chemical reaction that absorbs thermal. The applied heat helps break the intermolecular bonds between the water. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Therefore, boiling water is. Is Water Boiling Endothermic Or Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Is Water Boiling Endothermic Or Exothermic Boiling water is an endothermic process. Boiling water is an endothermic process that absorbs heat from the surroundings. This is mostly because heat must be applied in order for water to boil. An endothermic reaction is a chemical reaction that absorbs thermal. The absorption of heat during boiling results in a cooling effect. This means it absorbs heat from its. Is Water Boiling Endothermic Or Exothermic.
From loehrbtpf.blob.core.windows.net
Endothermic Reaction Thermal at John Maxey blog Is Water Boiling Endothermic Or Exothermic In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. An endothermic reaction is a chemical reaction that absorbs thermal. Because you are adding heat/energy, the reaction is endothermic. Factors such as atmospheric pressure,. This is mostly because heat must be applied in order for water to boil. Boiling water is. Is Water Boiling Endothermic Or Exothermic.
From manuallistbanderole.z13.web.core.windows.net
Energy Level Diagram For Endothermic Reaction Is Water Boiling Endothermic Or Exothermic Because you are adding heat/energy, the reaction is endothermic. Factors such as atmospheric pressure,. Boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Therefore, boiling water is an endothermic process. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat.. Is Water Boiling Endothermic Or Exothermic.
From loehrbtpf.blob.core.windows.net
Endothermic Reaction Thermal at John Maxey blog Is Water Boiling Endothermic Or Exothermic Because you are adding heat/energy, the reaction is endothermic. The process of boiling water is endothermic. An endothermic reaction is a chemical reaction that absorbs thermal. Therefore, boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Factors such as atmospheric pressure,. The absorption of heat during boiling results in. Is Water Boiling Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Is Water Boiling Endothermic Or Exothermic This means it absorbs heat from its surroundings. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. An endothermic reaction is a chemical reaction that absorbs thermal. Therefore, boiling water is an endothermic process. The absorption of heat during boiling results in a cooling effect. Because you are adding heat/energy,. Is Water Boiling Endothermic Or Exothermic.
From www.greelane.com
Contoh Tindak Balas Endotermik Is Water Boiling Endothermic Or Exothermic The applied heat helps break the intermolecular bonds between the water. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. This means it absorbs heat from its surroundings. Boiling water is an endothermic process that absorbs heat from the surroundings. Boiling water is an endothermic process. This is mostly because. Is Water Boiling Endothermic Or Exothermic.
From www.numerade.com
SOLVED Which of these is the TRUE statement? A) Combustion reactions Is Water Boiling Endothermic Or Exothermic Therefore, boiling water is an endothermic process. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Factors such as atmospheric pressure,. Boiling water is an endothermic process. This is mostly because heat must be applied in order for water to boil. The absorption of heat during boiling results in a. Is Water Boiling Endothermic Or Exothermic.
From www.tiktok.com
marquem Is Water Boiling Endothermic Or Exothermic Boiling water is an endothermic process. Factors such as atmospheric pressure,. Because you are adding heat/energy, the reaction is endothermic. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. This is mostly because heat must be. Is Water Boiling Endothermic Or Exothermic.
From www.tiktok.com
marquem Is Water Boiling Endothermic Or Exothermic In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. This means it absorbs heat from its surroundings. Factors such as atmospheric pressure,. Because you are adding heat/energy, the reaction is endothermic. The absorption of heat during boiling results in a cooling effect. Boiling water is an endothermic process. The process. Is Water Boiling Endothermic Or Exothermic.
From mungfali.com
Exothermic Vs Endothermic Diagram Is Water Boiling Endothermic Or Exothermic Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. This is because energy is required to break the hydrogen. This is mostly because heat must be applied in order for water to boil. This means it. Is Water Boiling Endothermic Or Exothermic.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Is Water Boiling Endothermic Or Exothermic In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. This means it absorbs heat from its surroundings. An endothermic reaction is a chemical reaction that absorbs thermal. This is because energy is required to break the. Is Water Boiling Endothermic Or Exothermic.
From sciencenotes.org
Endothermic Reactions Definition and Examples Is Water Boiling Endothermic Or Exothermic This is because energy is required to break the hydrogen. Because you are adding heat/energy, the reaction is endothermic. In thermodynamics, an endothermic process is one that absorbs heat from the surroundings, while an exothermic process releases heat. Boiling water is an endothermic process. The applied heat helps break the intermolecular bonds between the water. The process of boiling water. Is Water Boiling Endothermic Or Exothermic.