What Is Catalyst In Science . In homogeneous catalysis, catalysts are in the same phase as the reactants. They can either lower the activation energy of the reaction or change the mechanism of the reaction completely. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. They do chemically change during the reaction, but not permanently. This process is called catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Enzymes are biological catalysts that.
from www.alamy.com
An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. They can either lower the activation energy of the reaction or change the mechanism of the reaction completely. In homogeneous catalysis, catalysts are in the same phase as the reactants. A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst.
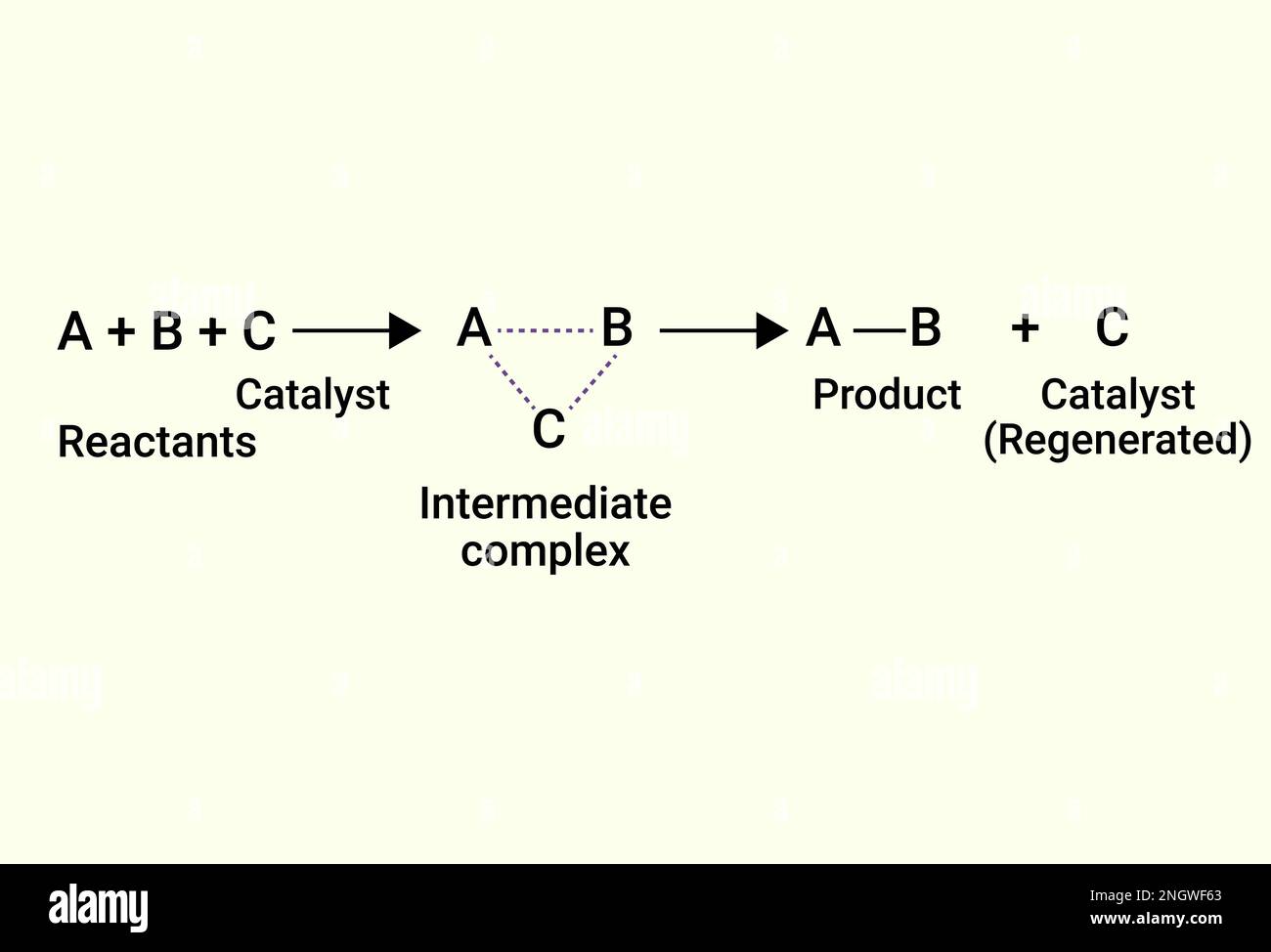
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy
What Is Catalyst In Science Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst is a substance which increases the rate of a reaction without being used up itself. In homogeneous catalysis, catalysts are in the same phase as the reactants. A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are biological catalysts that. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. They do chemically change during the reaction, but not permanently.
From www.tes.com
Catalysts lesson and experiment Teaching Resources What Is Catalyst In Science A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst, in turn, is a substance. What Is Catalyst In Science.
From 2012books.lardbucket.org
Catalysis What Is Catalyst In Science A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalyst, in chemistry, any substance that increases the rate of a. What Is Catalyst In Science.
From scitechdaily.com
Science Made Simple What Are Catalysts? What Is Catalyst In Science They do chemically change during the reaction, but not permanently. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a substance which increases the rate of a reaction without being used up itself. In homogeneous catalysis, catalysts are in the same. What Is Catalyst In Science.
From www.slideshare.net
Biology 2.4 What Is Catalyst In Science This process is called catalysis. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysis is defined. What Is Catalyst In Science.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2380033 What Is Catalyst In Science Enzymes are biological catalysts that. A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst,. What Is Catalyst In Science.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation What Is Catalyst In Science Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. In homogeneous catalysis, catalysts are in the same phase as the reactants. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. They can either lower the activation. What Is Catalyst In Science.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii What Is Catalyst In Science Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalysts allow a reaction to proceed via a pathway. What Is Catalyst In Science.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation, free download ID676158 What Is Catalyst In Science Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but.. What Is Catalyst In Science.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste What Is Catalyst In Science A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. Enzymes are biological catalysts that. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. A catalyst is not consumed by the reaction and it may participate in multiple reactions at. What Is Catalyst In Science.
From www.slideshare.net
Catalyst What Is Catalyst In Science Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do chemically change during the reaction, but not permanently. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance which increases the rate of a reaction without being used. What Is Catalyst In Science.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint What Is Catalyst In Science A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. In homogeneous catalysis, catalysts are in the same phase as the reactants. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in. What Is Catalyst In Science.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 What Is Catalyst In Science Enzymes are biological catalysts that. They do chemically change during the reaction, but not permanently. They can either lower the activation energy of the reaction or change the mechanism of the reaction completely. In homogeneous catalysis, catalysts are in the same phase as the reactants. A catalyst that is in a separate phase from the reactants is said to be. What Is Catalyst In Science.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID What Is Catalyst In Science Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Enzymes are biological catalysts that. A catalyst is. What Is Catalyst In Science.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of What Is Catalyst In Science Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Enzymes are biological catalysts that. A catalyst, in turn, is. What Is Catalyst In Science.
From www.researchgate.net
Catalyst development strategies. Schematic of various catalyst What Is Catalyst In Science In homogeneous catalysis, catalysts are in the same phase as the reactants. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. They can either lower the activation energy of the reaction or change the mechanism of. What Is Catalyst In Science.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint What Is Catalyst In Science In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Enzymes are biological catalysts that. A catalyst that is. What Is Catalyst In Science.
From www.eurekalert.org
Catalysis Illustration [IMAGE] EurekAlert! Science News Releases What Is Catalyst In Science A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Enzymes are biological catalysts that. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst that is in a separate phase from the reactants is said to. What Is Catalyst In Science.
From www.nagwa.com
Lesson Catalysts Nagwa What Is Catalyst In Science Enzymes are biological catalysts that. They do chemically change during the reaction, but not permanently. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. An example of heterogeneous catalysis is the use of. What Is Catalyst In Science.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B What Is Catalyst In Science In homogeneous catalysis, catalysts are in the same phase as the reactants. They do chemically change during the reaction, but not permanently. Enzymes are biological catalysts that. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst. What Is Catalyst In Science.
From www.catalystseurope.org
How are catalysts used? What Is Catalyst In Science A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Enzymes are biological catalysts that. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to. What Is Catalyst In Science.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 What Is Catalyst In Science A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. This process is called catalysis. A catalyst is a substance which increases the rate of a reaction without being used. What Is Catalyst In Science.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis What Is Catalyst In Science They do chemically change during the reaction, but not permanently. A catalyst is a substance which increases the rate of a reaction without being used up itself. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process. What Is Catalyst In Science.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst What Is Catalyst In Science Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. A catalyst is a chemical substance that affects. What Is Catalyst In Science.
From study.com
Catalyst Definition, Types & Function Lesson What Is Catalyst In Science Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in. What Is Catalyst In Science.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy What Is Catalyst In Science A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do chemically change during the reaction, but not permanently. In homogeneous catalysis, catalysts are in the same phase as the reactants. This process. What Is Catalyst In Science.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions What Is Catalyst In Science Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do chemically change during the reaction, but not permanently. This process is called catalysis. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst is not consumed by the reaction and it. What Is Catalyst In Science.
From www.youtube.com
Heterogeneous catalyst & catalysis 12th Std Chemistry Science What Is Catalyst In Science In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Enzymes are biological catalysts that. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalyst, in chemistry, any substance that increases the. What Is Catalyst In Science.
From www.wisegeek.org
What is a Catalyst? (with pictures) What Is Catalyst In Science Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. They can either lower the activation energy of the reaction or change the mechanism of the reaction completely. A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. Enzymes are biological catalysts that. Catalysts allow. What Is Catalyst In Science.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst What Is Catalyst In Science A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. In homogeneous catalysis, catalysts are in the same phase as the reactants. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a chemical substance that affects the rate of a chemical reaction by. What Is Catalyst In Science.
From www.youtube.com
How does a CATALYST work ? YouTube What Is Catalyst In Science They can either lower the activation energy of the reaction or change the mechanism of the reaction completely. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Enzymes are biological catalysts that. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In homogeneous catalysis, catalysts are in. What Is Catalyst In Science.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst What Is Catalyst In Science Enzymes are biological catalysts that. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. What Is Catalyst In Science.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub What Is Catalyst In Science Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Enzymes are biological catalysts that. In homogeneous catalysis, catalysts are in the same phase as the reactants. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. A catalyst is a chemical. What Is Catalyst In Science.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram What Is Catalyst In Science They do chemically change during the reaction, but not permanently. This process is called catalysis. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Enzymes are biological catalysts that. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance. What Is Catalyst In Science.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica What Is Catalyst In Science Enzymes are biological catalysts that. A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. An example of heterogeneous catalysis. What Is Catalyst In Science.
From www.youtube.com
Demonstration of a Simple Catalyst Experiment YouTube What Is Catalyst In Science An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a chemical substance that affects. What Is Catalyst In Science.