Iodine Zinc Exothermic Reaction . the exothermic reaction between iodine and zinc.more. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. let's make the zinc react with the iodine, thereby obtaining zinc. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. The experiment can be extended to show the decomposition of a compound into its elements. Zn + i 2 → zni 2. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. zinc + iodine = zinc iodide. reaction of zinc and iodine. Adding drops of water to the mixture. Granular zinc and powdered iodine are mixed together. reaction of zinc with iodine. reaction of zinc and iodine.
from www.tutormyself.com
Zn + i 2 → zni 2. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. reaction of zinc and iodine. reaction of zinc with iodine. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Adding drops of water to the mixture. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. zinc + iodine = zinc iodide. reaction of zinc and iodine.
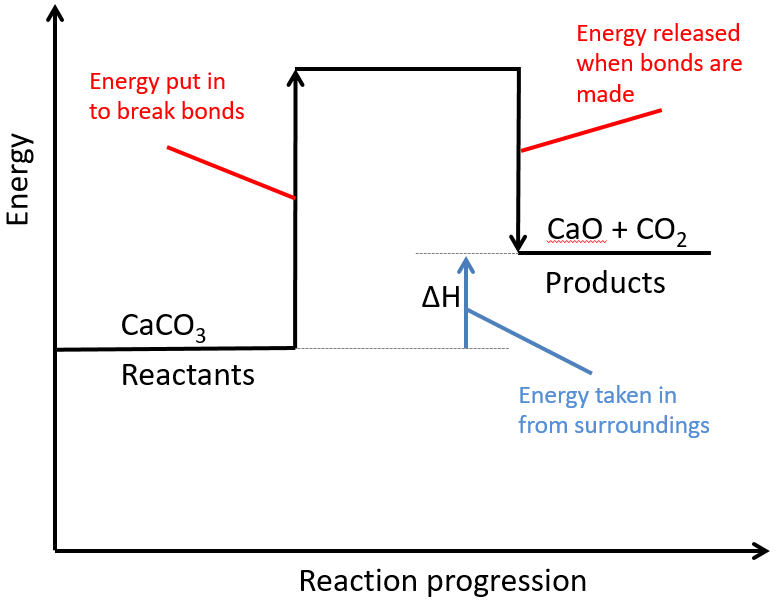
314 (Triple only) draw and explain reaction profile diagrams showing
Iodine Zinc Exothermic Reaction an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. reaction of zinc and iodine. reaction of zinc with iodine. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. let's make the zinc react with the iodine, thereby obtaining zinc. The experiment can be extended to show the decomposition of a compound into its elements. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Zn + i 2 → zni 2. reaction of zinc and iodine. Adding drops of water to the mixture. the exothermic reaction between iodine and zinc.more. zinc + iodine = zinc iodide. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Granular zinc and powdered iodine are mixed together. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor.
From brainly.in
13. in an experiment 20 g of zinc was reacted with 20 g of iodine in a Iodine Zinc Exothermic Reaction The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. let's make the zinc react with the iodine, thereby obtaining zinc. the exothermic reaction between iodine and zinc.more. Adding. Iodine Zinc Exothermic Reaction.
From www.toppr.com
In the reaction between zinc and iodine, zinc iodide is formed. Which Iodine Zinc Exothermic Reaction reaction of zinc and iodine. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. Zn + i 2 → zni 2. zinc + iodine = zinc iodide. reaction of zinc and iodine. The experiment can be extended to show the decomposition of a compound. Iodine Zinc Exothermic Reaction.
From www.worksheetsplanet.com
What is an Exothermic Reaction Definition and Example Iodine Zinc Exothermic Reaction let's make the zinc react with the iodine, thereby obtaining zinc. Adding drops of water to the mixture. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. the exothermic reaction between iodine and zinc.more. The experiment can be extended to show the decomposition of a. Iodine Zinc Exothermic Reaction.
From www.youtube.com
Reaction of Iodine and Zinc vigorous exothermic reaction YouTube Iodine Zinc Exothermic Reaction reaction of zinc with iodine. reaction of zinc and iodine. Adding drops of water to the mixture. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. let's make the zinc react with the iodine, thereby obtaining zinc. Granular zinc and powdered iodine are. Iodine Zinc Exothermic Reaction.
From partdiagramaminabakery5v.z14.web.core.windows.net
Exothermic And Endothermic Diagrams Iodine Zinc Exothermic Reaction Zn + i 2 → zni 2. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. let's make the zinc react with the iodine, thereby obtaining zinc. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. The experiment can be extended to. Iodine Zinc Exothermic Reaction.
From mmeijerchemreactions.blogspot.com
Chemical Reactions Zinc And Iodine Lab Iodine Zinc Exothermic Reaction Granular zinc and powdered iodine are mixed together. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. let's make the zinc react with the iodine, thereby obtaining zinc. Zn + i 2 → zni 2. Adding drops of water to the mixture. The addition of a few ml of water. Iodine Zinc Exothermic Reaction.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Iodine Zinc Exothermic Reaction reaction of zinc with iodine. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. the exothermic reaction between iodine and zinc.more. Zn + i 2 → zni 2. The experiment can be extended to show the decomposition of a compound into its elements. Adding drops of water to the. Iodine Zinc Exothermic Reaction.
From wiredatatisse56wr.z22.web.core.windows.net
Labelled Diagram Of Exothermic Reaction Iodine Zinc Exothermic Reaction the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. reaction of zinc with iodine. Granular zinc and powdered iodine are mixed together. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. an exothermic redox reaction occurs, forming zinc iodide, which can. Iodine Zinc Exothermic Reaction.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Iodine Zinc Exothermic Reaction an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. reaction of zinc and iodine. Zn + i 2 → zni 2. let's make the zinc react with the iodine, thereby obtaining zinc. Granular zinc and powdered iodine are mixed together. zinc + iodine = zinc iodide. The experiment can. Iodine Zinc Exothermic Reaction.
From www.sciencephoto.com
Zinc reacts with iodine Stock Image C044/0436 Science Photo Library Iodine Zinc Exothermic Reaction zinc + iodine = zinc iodide. reaction of zinc and iodine. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. the exothermic reaction between iodine and. Iodine Zinc Exothermic Reaction.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Iodine Zinc Exothermic Reaction Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. reaction of zinc with iodine. let's make the zinc react with the iodine, thereby obtaining zinc. reaction of zinc and iodine. reaction of zinc and iodine. the reaction of zinc metal with. Iodine Zinc Exothermic Reaction.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Iodine Zinc Exothermic Reaction The experiment can be extended to show the decomposition of a compound into its elements. zinc + iodine = zinc iodide. Zn + i 2 → zni 2. reaction of zinc and iodine. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. reaction. Iodine Zinc Exothermic Reaction.
From www.tutormyself.com
314 (Triple only) draw and explain reaction profile diagrams showing Iodine Zinc Exothermic Reaction Zn + i 2 → zni 2. The experiment can be extended to show the decomposition of a compound into its elements. Granular zinc and powdered iodine are mixed together. reaction of zinc and iodine. let's make the zinc react with the iodine, thereby obtaining zinc. Zn + i = zni2 is a synthesis reaction where one mole. Iodine Zinc Exothermic Reaction.
From fphoto.photoshelter.com
science chemistry exothermic reaction iodine zinc Fundamental Iodine Zinc Exothermic Reaction reaction of zinc with iodine. the exothermic reaction between iodine and zinc.more. reaction of zinc and iodine. reaction of zinc and iodine. let's make the zinc react with the iodine, thereby obtaining zinc. Granular zinc and powdered iodine are mixed together. Zn + i = zni2 is a synthesis reaction where one mole of zinc. Iodine Zinc Exothermic Reaction.
From www.youtube.com
Chemistry experiment 14 Reaction between iodine and zinc YouTube Iodine Zinc Exothermic Reaction an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. reaction of zinc and iodine. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn]. Iodine Zinc Exothermic Reaction.
From studylib.net
Reaction of Zinc and Iodine Department of Chemistry Iodine Zinc Exothermic Reaction the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. the exothermic. Iodine Zinc Exothermic Reaction.
From mavink.com
Endothermic And Exothermic Reactions Examples Iodine Zinc Exothermic Reaction the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. zinc + iodine = zinc iodide. reaction of zinc and iodine. Zn + i 2 → zni 2. The addition of a few. Iodine Zinc Exothermic Reaction.
From www.numerade.com
SOLVED Consider the reaction between an iodine solution and starch Iodine Zinc Exothermic Reaction reaction of zinc and iodine. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. Zn + i 2 → zni 2. reaction of zinc and iodine. The experiment can be extended to show the decomposition of a compound into its elements. zinc + iodine. Iodine Zinc Exothermic Reaction.
From www.youtube.com
Iodine & Zinc Reaction YouTube Iodine Zinc Exothermic Reaction let's make the zinc react with the iodine, thereby obtaining zinc. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. zinc + iodine = zinc iodide. Granular zinc and powdered iodine are. Iodine Zinc Exothermic Reaction.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Iodine Zinc Exothermic Reaction The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. The experiment can be extended to show the decomposition of a compound into its elements. Adding drops of water to the mixture. let's make the zinc react with the iodine, thereby obtaining zinc. reaction of zinc. Iodine Zinc Exothermic Reaction.
From www.tutormyself.com
314 (Triple only) draw and explain reaction profile diagrams showing Iodine Zinc Exothermic Reaction Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. zinc + iodine = zinc iodide. reaction of zinc and iodine. Adding drops of water to the. Iodine Zinc Exothermic Reaction.
From oseusurf.blogspot.com
Zinc Reacts With Aqueous Sulfuric Acid To Form Hydrogen Gas 20+ Pages Iodine Zinc Exothermic Reaction reaction of zinc and iodine. reaction of zinc and iodine. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. zinc + iodine = zinc iodide. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. the reaction of zinc metal. Iodine Zinc Exothermic Reaction.
From learningschoolexecutry.z14.web.core.windows.net
Everyday Examples Of Exothermic Reactions Iodine Zinc Exothermic Reaction an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. reaction of zinc and iodine. Granular zinc and powdered iodine are mixed together. The addition of a few ml of water to a mixture of elemental zinc and iodine results in. The addition of a few ml of water to a mixture. Iodine Zinc Exothermic Reaction.
From fphoto.photoshelter.com
science chemistry exothermic reaction iodine zinc Fundamental Iodine Zinc Exothermic Reaction the exothermic reaction between iodine and zinc.more. Granular zinc and powdered iodine are mixed together. let's make the zinc react with the iodine, thereby obtaining zinc. reaction of zinc and iodine. Zn + i 2 → zni 2. zinc + iodine = zinc iodide. reaction of zinc with iodine. The addition of a few ml. Iodine Zinc Exothermic Reaction.
From edu-rsc-org-ssl.oca.korea.ac.kr
Exothermic redox reaction of zinc with iodine Experiment RSC Education Iodine Zinc Exothermic Reaction zinc + iodine = zinc iodide. Granular zinc and powdered iodine are mixed together. the exothermic reaction between iodine and zinc.more. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. reaction of zinc and iodine. Zn + i = zni2 is a synthesis reaction. Iodine Zinc Exothermic Reaction.
From fphoto.photoshelter.com
science chemistry exothermic reaction iodine zinc Fundamental Iodine Zinc Exothermic Reaction Zn + i 2 → zni 2. Granular zinc and powdered iodine are mixed together. Adding drops of water to the mixture. zinc + iodine = zinc iodide. let's make the zinc react with the iodine, thereby obtaining zinc. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. The addition. Iodine Zinc Exothermic Reaction.
From www.sciencephoto.com
Zinc reacts with iodine, 1 of 5 Stock Image C044/0440 Science Iodine Zinc Exothermic Reaction Zn + i 2 → zni 2. zinc + iodine = zinc iodide. Granular zinc and powdered iodine are mixed together. reaction of zinc and iodine. The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. let's make the zinc react with the iodine, thereby. Iodine Zinc Exothermic Reaction.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Exothermic metal displacement reactions Experiment RSC Education Iodine Zinc Exothermic Reaction the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. The experiment can be extended to show the decomposition of a compound into its elements. Zn + i 2 → zni 2. zinc + iodine = zinc iodide. The addition of a few ml of water to a mixture of elemental zinc. Iodine Zinc Exothermic Reaction.
From www.youtube.com
Zinc and iodine exothermic reaction. Creating Zinciodide solution for Iodine Zinc Exothermic Reaction The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. reaction of zinc and iodine. the exothermic reaction between iodine and zinc.more. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. zinc + iodine = zinc iodide.. Iodine Zinc Exothermic Reaction.
From fphoto.photoshelter.com
science chemistry exothermic reaction iodine zinc Fundamental Iodine Zinc Exothermic Reaction Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Granular zinc and powdered iodine are mixed together. Zn + i 2 → zni 2. the exothermic reaction between iodine and zinc.more. reaction of zinc and iodine. The experiment can be extended to show the. Iodine Zinc Exothermic Reaction.
From schematicorensano5824i.z19.web.core.windows.net
Exothermic And Endothermic Diagrams Iodine Zinc Exothermic Reaction The addition of a few ml of water to a mixture of elemental zinc and iodine results in. reaction of zinc and iodine. The experiment can be extended to show the decomposition of a compound into its elements. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. The addition of a. Iodine Zinc Exothermic Reaction.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.21 Practical Investigate Reactions Between Iodine Zinc Exothermic Reaction Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. the exothermic reaction between iodine and zinc.more. reaction of zinc and iodine. let's make the zinc react with the iodine, thereby obtaining zinc. Zn + i 2 → zni 2. Granular zinc and powdered. Iodine Zinc Exothermic Reaction.
From telegra.ph
Endothermic energy profile diagram activation Telegraph Iodine Zinc Exothermic Reaction let's make the zinc react with the iodine, thereby obtaining zinc. reaction of zinc and iodine. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles. Iodine Zinc Exothermic Reaction.
From www.sciencephoto.com
Zinc reacts with iodine, 3 of 5 Stock Image C044/0438 Science Iodine Zinc Exothermic Reaction zinc + iodine = zinc iodide. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. Zn + i 2 → zni 2. reaction of zinc and iodine. Granular zinc and powdered iodine are mixed together. the exothermic reaction between iodine and zinc.more. Zn + i = zni2 is a. Iodine Zinc Exothermic Reaction.
From www.toppr.com
In the reaction between zinc and iodine , zinc iodide is formed . What Iodine Zinc Exothermic Reaction The addition of a few ml of water to a mixture of elemental zinc and iodine results in evolution of purple vapor. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. reaction of zinc with iodine. The addition of a few ml of water to a mixture of elemental zinc and. Iodine Zinc Exothermic Reaction.