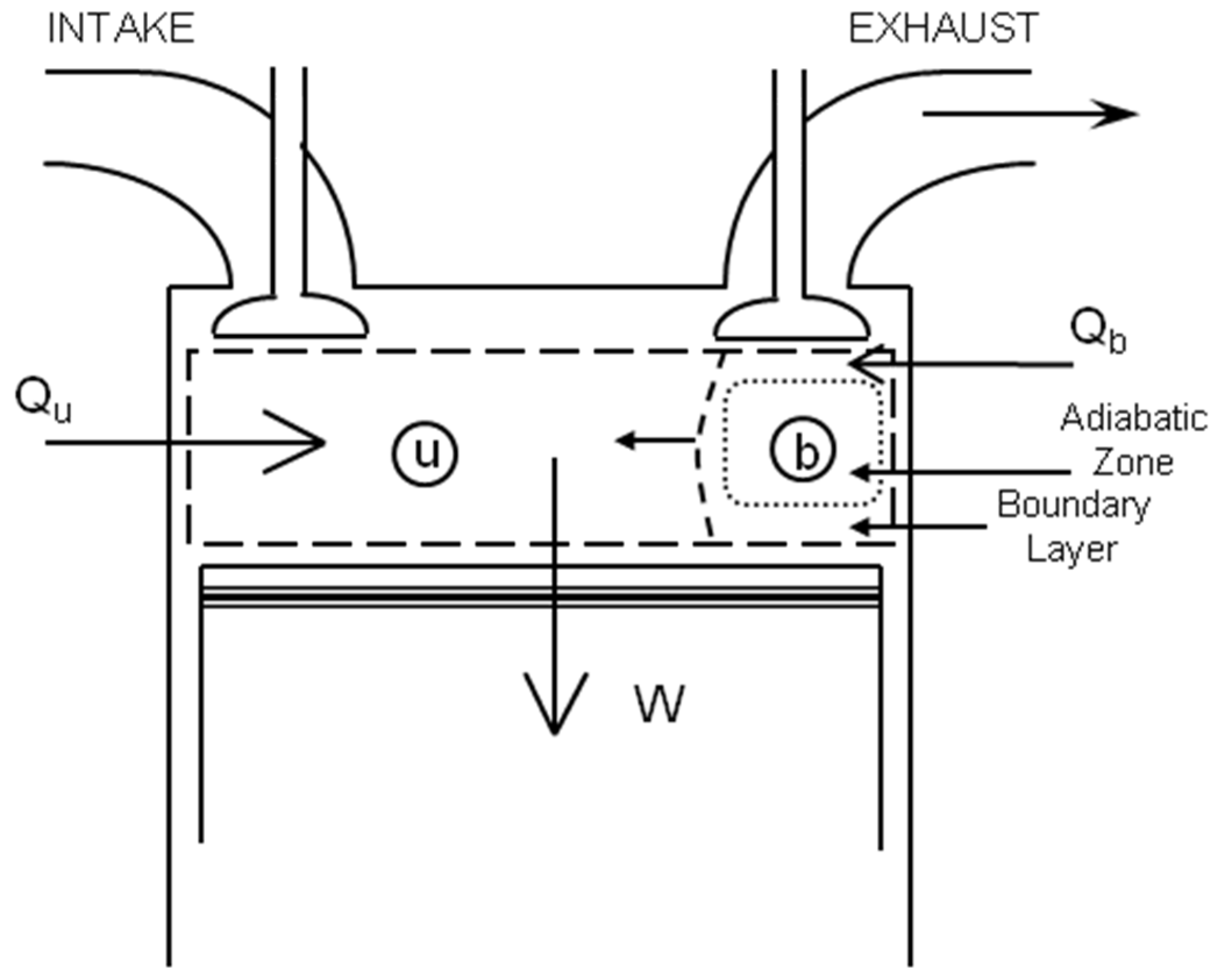
An Engine That Destroys Entropy Is . Another form of the second law of thermodynamics states that the total entropy of. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. Entropy is the loss of energy available to do work. It introduces the concept of entropy, which is. It takes energy to decrease entropy. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The second law of thermodynamics is a fundamental principle in physics and engineering. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases.
from www.mdpi.com
The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. It takes energy to decrease entropy. Entropy is the loss of energy available to do work. Another form of the second law of thermodynamics states that the total entropy of. It introduces the concept of entropy, which is. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The second law of thermodynamics is a fundamental principle in physics and engineering. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the.
Inventions Free FullText The Thermodynamics of Internal Combustion
An Engine That Destroys Entropy Is The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. Entropy is the loss of energy available to do work. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. It introduces the concept of entropy, which is. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. It takes energy to decrease entropy. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: Another form of the second law of thermodynamics states that the total entropy of. The second law of thermodynamics is a fundamental principle in physics and engineering. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the.
From www.youtube.com
Entropy change for Reversible process, & irreversible process, Heat An Engine That Destroys Entropy Is A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. Another form of. An Engine That Destroys Entropy Is.
From philschatz.com
The First Law of Thermodynamics and Some Simple Processes · Physics An Engine That Destroys Entropy Is The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. Entropy is the loss of energy available to do work. Another form of the second law of thermodynamics states that the total entropy. An Engine That Destroys Entropy Is.
From learncheme.com
entropyconceptest1 LearnChemE An Engine That Destroys Entropy Is The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. It introduces the concept of entropy, which is. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The second law of thermodynamics is a fundamental principle in physics and engineering. Another form of the second law of. An Engine That Destroys Entropy Is.
From guidemanualgiver.z21.web.core.windows.net
Thermodynamics Of A Heat Engine An Engine That Destroys Entropy Is The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The second law of thermodynamics is a fundamental principle in physics and engineering. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. The second law of thermodynamics says that, as time goes forward,. An Engine That Destroys Entropy Is.
From manuallibrarystarkest.z13.web.core.windows.net
Heat Engine Thermodynamic Cycle An Engine That Destroys Entropy Is Entropy is the loss of energy available to do work. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. It takes energy to decrease entropy. Any heat engine operating between the same two temperatures whose efficiency is. An Engine That Destroys Entropy Is.
From www.chegg.com
Solved Topic Chapter 18 Engines of Entropy What kind of An Engine That Destroys Entropy Is The second law of thermodynamics is a fundamental principle in physics and engineering. It takes energy to decrease entropy. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily. An Engine That Destroys Entropy Is.
From guidediagrammanlier.z21.web.core.windows.net
Thermodynamics Of A Heat Engine An Engine That Destroys Entropy Is A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The second law of thermodynamics is a fundamental principle in physics and engineering. The carnot engine model. An Engine That Destroys Entropy Is.
From www.doubtnut.com
The temperatureentropy diagram of a reversible engine cycle is given An Engine That Destroys Entropy Is Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: Entropy is the loss of energy available to do work. It introduces the concept of entropy, which. An Engine That Destroys Entropy Is.
From wiringfixtaproots.z21.web.core.windows.net
Thermodynamics In Car Engines An Engine That Destroys Entropy Is It takes energy to decrease entropy. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. The second law of thermodynamics is a fundamental principle in physics and engineering. Another form of the second law of thermodynamics states that the total entropy of. It introduces the concept of entropy, which is.. An Engine That Destroys Entropy Is.
From sciencekahani.blogspot.com
Science Kahani Thermodynamics(Auto cycle, Diesel cycle,Brayton cycle An Engine That Destroys Entropy Is It takes energy to decrease entropy. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: Entropy is the loss of energy available to do work. The second law of thermodynamics is a fundamental principle in physics and engineering. Any heat engine operating between the. An Engine That Destroys Entropy Is.
From www.mdpi.com
Entropy Free FullText Comparison Based on Exergetic Analyses of An Engine That Destroys Entropy Is The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: It introduces the concept of entropy, which is. Entropy is the loss of energy available to do work. The second law. An Engine That Destroys Entropy Is.
From www.aparat.com
Solution Manual for Energy, Entropy and Engines Sanjeev Chandra An Engine That Destroys Entropy Is The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Entropy is the loss of energy available to do work. It introduces the concept of entropy, which is. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The carnot engine model was graphically expanded by benoît paul. An Engine That Destroys Entropy Is.
From wiremanualcolucci.z13.web.core.windows.net
Enthalpy Diagram Entropy Jet Engine An Engine That Destroys Entropy Is Another form of the second law of thermodynamics states that the total entropy of. The second law of thermodynamics is a fundamental principle in physics and engineering. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The second law of thermodynamics says that, as. An Engine That Destroys Entropy Is.
From courses.lumenlearning.com
Introduction to the Second Law of Thermodynamics Heat Engines and An Engine That Destroys Entropy Is The second law of thermodynamics is a fundamental principle in physics and engineering. It takes energy to decrease entropy. It introduces the concept of entropy, which is. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$. An Engine That Destroys Entropy Is.
From www.eigenplus.com
eigenplus An Engine That Destroys Entropy Is A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. It introduces the concept of entropy, which is. It takes energy to decrease entropy. Entropy is the loss of energy available. An Engine That Destroys Entropy Is.
From www.mdpi.com
Entropy Free FullText Effect of FiniteSize Heat Source’s Heat An Engine That Destroys Entropy Is A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Entropy is the loss of energy available to do work. It introduces the concept of entropy, which. An Engine That Destroys Entropy Is.
From www.dreamstime.com
Second Law of Thermodynamics Infographic Diagram Stock Vector An Engine That Destroys Entropy Is The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The second law of thermodynamics is a fundamental principle in physics and engineering. It takes energy to decrease entropy. It introduces the concept of entropy, which is. A. An Engine That Destroys Entropy Is.
From slideplayer.com
Heat Engines, Entropy, & the 2nd Law of Thermodynamics ppt download An Engine That Destroys Entropy Is A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: It takes energy to decrease entropy. Entropy is the loss of energy available to do work. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. It. An Engine That Destroys Entropy Is.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 6, pt 3 of 4 Heat Engines An Engine That Destroys Entropy Is Another form of the second law of thermodynamics states that the total entropy of. It introduces the concept of entropy, which is. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. It takes energy to decrease entropy. A heat engine is a device which takes a thermodynamic system through a repeated. An Engine That Destroys Entropy Is.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 An Engine That Destroys Entropy Is It takes energy to decrease entropy. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The second law of thermodynamics is a fundamental principle in physics and engineering. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Entropy is the loss of energy available to do. An Engine That Destroys Entropy Is.
From www.mdpi.com
Entropy Free FullText Association of FiniteTime Thermodynamics An Engine That Destroys Entropy Is It introduces the concept of entropy, which is. Entropy is the loss of energy available to do work. The second law of thermodynamics is a fundamental principle in physics and engineering. It takes energy to decrease entropy. Another form of the second law of thermodynamics states that the total entropy of. A heat engine is a device which takes a. An Engine That Destroys Entropy Is.
From www.slideserve.com
PPT Entropy in the Real World Engines PowerPoint Presentation, free An Engine That Destroys Entropy Is The second law of thermodynamics is a fundamental principle in physics and engineering. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$. An Engine That Destroys Entropy Is.
From diagramlibraryoily.z5.web.core.windows.net
Heat Engine Diagram Thermodynamics An Engine That Destroys Entropy Is Entropy is the loss of energy available to do work. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: It takes energy to decrease entropy.. An Engine That Destroys Entropy Is.
From www.vrogue.co
Ppt Siklus Carnot vrogue.co An Engine That Destroys Entropy Is Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. Entropy is the loss of energy available to do work. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The second law of thermodynamics is a fundamental principle in physics and engineering. The net result of such. An Engine That Destroys Entropy Is.
From www.mdpi.com
Entropy Free FullText Performance of Quantum Heat Engines Enhanced An Engine That Destroys Entropy Is It takes energy to decrease entropy. Entropy is the loss of energy available to do work. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. Another. An Engine That Destroys Entropy Is.
From slideplayer.com
Entropy and the Second Law of Thermodynamics ppt download An Engine That Destroys Entropy Is Another form of the second law of thermodynamics states that the total entropy of. It introduces the concept of entropy, which is. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. It takes energy to decrease entropy. Any heat engine operating between the same two temperatures whose efficiency is less. An Engine That Destroys Entropy Is.
From www.studocu.com
Chapter 20 Entropy Engines Chapter 20 of t ↑ some heatlost TH ①A An Engine That Destroys Entropy Is A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. It introduces the. An Engine That Destroys Entropy Is.
From guidelibunveracity.z21.web.core.windows.net
Entropy And Temp Relation An Engine That Destroys Entropy Is It introduces the concept of entropy, which is. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. The carnot engine model was graphically expanded by benoît. An Engine That Destroys Entropy Is.
From manuallibrarystarkest.z13.web.core.windows.net
Heat Engine Thermodynamic Cycle An Engine That Destroys Entropy Is The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. Another form of the second law of thermodynamics states that the total entropy of. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be represented as a succession of equilibrium states: The net. An Engine That Destroys Entropy Is.
From guidelibunveracity.z21.web.core.windows.net
Heat Engine Diagram Thermodynamics An Engine That Destroys Entropy Is The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. The second law of thermodynamics says that, as time goes forward, entropy (disorder) increases. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. Another form of the second law of thermodynamics states. An Engine That Destroys Entropy Is.
From www.mdpi.com
Entropy Free FullText FiniteTime Thermodynamic Model for An Engine That Destroys Entropy Is The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Entropy is the loss of energy available to do work. Another form of the second law of thermodynamics states that the total entropy of. A heat engine is a device which takes a thermodynamic system through a repeated cycle which can be. An Engine That Destroys Entropy Is.
From www.mdpi.com
Entropy Free FullText Stochastic Stirling Engine Operating in An Engine That Destroys Entropy Is Another form of the second law of thermodynamics states that the total entropy of. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. It takes energy to decrease entropy. The second law of thermodynamics is a fundamental principle in physics and engineering. The second law of thermodynamics says that, as. An Engine That Destroys Entropy Is.
From www.eigenplus.com
Carnot Efficiency Formula, Derivation & Explanation eigenplus An Engine That Destroys Entropy Is The net result of such a cyclic process is to convert heat into mechanical work, or vice versa. Entropy is the loss of energy available to do work. It takes energy to decrease entropy. The second law of thermodynamics is a fundamental principle in physics and engineering. The second law of thermodynamics says that, as time goes forward, entropy (disorder). An Engine That Destroys Entropy Is.
From www.mdpi.com
Inventions Free FullText The Thermodynamics of Internal Combustion An Engine That Destroys Entropy Is It introduces the concept of entropy, which is. It takes energy to decrease entropy. The second law of thermodynamics is a fundamental principle in physics and engineering. Entropy is the loss of energy available to do work. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. A heat engine is a. An Engine That Destroys Entropy Is.
From www.reddit.com
is this possible guys 🤣🤣 any problem thermodynamics okbuddyphd An Engine That Destroys Entropy Is It takes energy to decrease entropy. Any heat engine operating between the same two temperatures whose efficiency is less than $e_c$ necessarily increases the. Entropy is the loss of energy available to do work. The carnot engine model was graphically expanded by benoît paul émile clapeyron in 1834 and mathematically explored by rudolf. A heat engine is a device which. An Engine That Destroys Entropy Is.