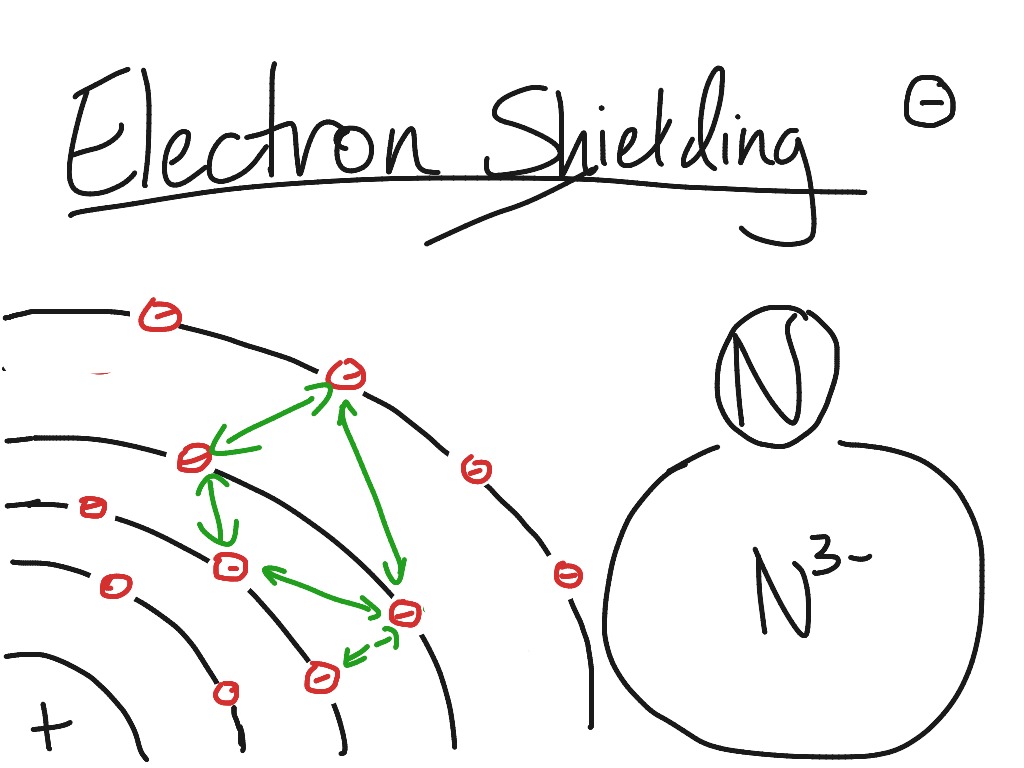
Electron Cloud Shielding Effect . The amount of charge felt by an. In the first case the local magnetic. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.
from www.showme.com
(a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons further from the nucleus (red). In the first case the local magnetic. The amount of charge felt by an. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.
Electron shielding Science, Chemistry, Electrons, electron shielding
Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The amount of charge felt by an. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. In the first case the local magnetic. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Electron Cloud Shielding Effect (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. In the first case the local magnetic. The amount of charge felt by an. The shielding. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect In the first case the local magnetic. The amount of charge felt by an. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. Electrons further. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The amount of charge felt by an. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the. Electron Cloud Shielding Effect.
From www.mri-q.com
MR thermography Questions and Answers in MRI Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The amount of charge felt by an. In the first case the local magnetic. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in. Electron Cloud Shielding Effect.
From www.toppr.com
How are shielding effect and atomic radius related? Electron Cloud Shielding Effect The amount of charge felt by an. Electrons further from the nucleus (red). In the first case the local magnetic. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons further from the nucleus (red). In the first case the local magnetic. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\). Electron Cloud Shielding Effect.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick Electron Cloud Shielding Effect (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons further from the nucleus (red). The amount of charge felt by an. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. In the first case the local magnetic. Electrons further from the nucleus. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free Electron Cloud Shielding Effect In the first case the local magnetic. The amount of charge felt by an. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. (a) the interior electron cloud (light. Electron Cloud Shielding Effect.
From www.toppr.com
The ionization energies from Ga to Tl do not decrease due to Electron Cloud Shielding Effect In the first case the local magnetic. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. Electrons further from the nucleus (red). (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The amount of. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free Electron Cloud Shielding Effect Electrons further from the nucleus (red). The amount of charge felt by an. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The shielding effect. Electron Cloud Shielding Effect.
From www.youtube.com
Shielding Effect YouTube Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons further from the nucleus (red). (a) the interior electron cloud (light. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. Electrons further from the nucleus (red). The amount of charge felt by. Electron Cloud Shielding Effect.
From www.researchgate.net
Electron cloudinduced related effects. Download Scientific Diagram Electron Cloud Shielding Effect (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint Electron Cloud Shielding Effect In the first case the local magnetic. Electrons further from the nucleus (red). The amount of charge felt by an. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons further from the nucleus (red). In the first case the local magnetic. Electrons. Electron Cloud Shielding Effect.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding Electron Cloud Shielding Effect (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The amount of charge felt by an. In the first case the local magnetic. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons further from the nucleus (red). Electrons in an \(s\) orbital can shield \(p\). Electron Cloud Shielding Effect.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 Electron Cloud Shielding Effect The amount of charge felt by an. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. In the first case the local magnetic. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. Electrons further. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT Electron Cloud Detection PowerPoint Presentation, free download Electron Cloud Shielding Effect Electrons further from the nucleus (red). The amount of charge felt by an. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect In the first case the local magnetic. The amount of charge felt by an. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because. Electron Cloud Shielding Effect.
From www.pinterest.com
SHIELDING EFFECT Physical chemistry, Chemistry notes, Physics formulas Electron Cloud Shielding Effect Electrons further from the nucleus (red). The amount of charge felt by an. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. In. Electron Cloud Shielding Effect.
From chemistry.stackexchange.com
chemistry My book's claim about the shielding effect of s,p Electron Cloud Shielding Effect In the first case the local magnetic. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The amount of charge felt. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 Electron Cloud Shielding Effect In the first case the local magnetic. Electrons further from the nucleus (red). Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The amount of. Electron Cloud Shielding Effect.
From animalia-life.club
Shielding Effect Trend Electron Cloud Shielding Effect The amount of charge felt by an. In the first case the local magnetic. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in. Electron Cloud Shielding Effect.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The amount of charge felt by an. Electrons further from the nucleus. Electron Cloud Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Cloud Shielding Effect The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons further from the nucleus (red). The amount of charge felt by an. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons. Electron Cloud Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting Electron Cloud Shielding Effect The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. In the first case the local magnetic. Electrons further from the nucleus. Electron Cloud Shielding Effect.
From byjus.com
What is shielding and deshielding in NMR? Give an example? Electron Cloud Shielding Effect The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. In the first case the local magnetic. (a) the interior electron cloud. Electron Cloud Shielding Effect.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. In the first case the local magnetic. Electrons further from the nucleus. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2617702 Electron Cloud Shielding Effect Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. In the first case the local magnetic. The amount of charge felt. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 Electron Cloud Shielding Effect Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. In the first case the local magnetic. The. Electron Cloud Shielding Effect.
From www.slideserve.com
PPT Emittance growth induced by electron cloud in proton storage Electron Cloud Shielding Effect In the first case the local magnetic. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. Electrons further from the nucleus. Electron Cloud Shielding Effect.
From scienceinfo.com
Shielding effect Electron Cloud Shielding Effect In the first case the local magnetic. Electrons further from the nucleus (red). The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons. Electron Cloud Shielding Effect.