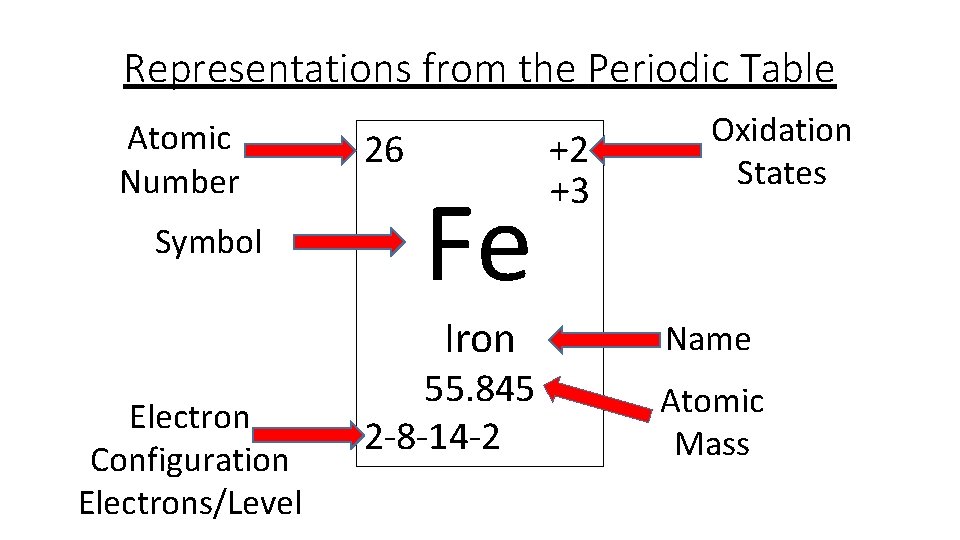
Iron Electrons Element . It describes how electrons are distributed among the various atomic. This configuration reveals iron’s chemical behavior. The electron configuration of iron is [ar] 3d6 4s2. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is a chemical element with symbol fe and atomic number 26. Iron can form various oxidation. It is by mass the most. It’s a transition metal from group 8 of the periodic table’s first transition series. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron is a chemical element with atomic number. It is a metal in the first transition series. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Fe, fe2+, and fe3+ electron.
from modernizemodest1712.blogspot.com
Fe, fe2+, and fe3+ electron. This configuration reveals iron’s chemical behavior. Iron is a chemical element with atomic number. The electron configuration of iron is [ar] 3d6 4s2. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It is a metal in the first transition series. It describes how electrons are distributed among the various atomic. Iron can form various oxidation. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. It is by mass the most.
40 orbital diagram for fe Diagram For You
Iron Electrons Element This configuration reveals iron’s chemical behavior. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Fe, fe2+, and fe3+ electron. It’s a transition metal from group 8 of the periodic table’s first transition series. It is by mass the most. Iron can form various oxidation. Iron is a chemical element with atomic number. This configuration reveals iron’s chemical behavior. The electron configuration of iron is [ar] 3d6 4s2. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron is a chemical element with symbol fe and atomic number 26. It is a metal in the first transition series. It describes how electrons are distributed among the various atomic. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Electrons Element It’s a transition metal from group 8 of the periodic table’s first transition series. It is a metal in the first transition series. Iron is a chemical element with atomic number. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron can form various oxidation. It is by mass the most. It describes. Iron Electrons Element.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron Electrons Element Fe, fe2+, and fe3+ electron. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The electron configuration of iron is [ar] 3d6 4s2. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. It describes how electrons are distributed among the various atomic. It’s a transition metal from. Iron Electrons Element.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Electrons Element The chemical element iron has the atomic number 26 and the symbol fe (from latin: Fe, fe2+, and fe3+ electron. It describes how electrons are distributed among the various atomic. Iron can form various oxidation. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron is a chemical element with symbol fe and. Iron Electrons Element.
From www.alamy.com
Fe Iron, Periodic Table of the Elements, Shell Structure of Iron Iron Electrons Element The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron is a chemical element with atomic number. It is a metal in the first transition series. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It is by mass the most. Fe, fe2+, and fe3+ electron. This. Iron Electrons Element.
From www.britannica.com
Iron Element, Occurrence, Uses, Properties, & Compounds Britannica Iron Electrons Element Iron is a chemical element with symbol fe and atomic number 26. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Fe, fe2+, and fe3+ electron. It’s a transition metal from group 8 of the periodic table’s first transition series. It is a metal in the first transition series. It is by mass the. Iron Electrons Element.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic Iron Electrons Element Iron is a chemical element with symbol fe and atomic number 26. It is by mass the most. The electron configuration of iron is [ar] 3d6 4s2. Fe, fe2+, and fe3+ electron. It’s a transition metal from group 8 of the periodic table’s first transition series. It describes how electrons are distributed among the various atomic. When we write the. Iron Electrons Element.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Electrons Element Iron can form various oxidation. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. This configuration reveals iron’s chemical behavior. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. It describes how electrons are distributed among the various atomic. It is a metal. Iron Electrons Element.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Electrons Element It is by mass the most. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron can form various oxidation. The electron configuration of iron is [ar] 3d6 4s2. Iron is a chemical element with symbol fe and atomic number 26. This configuration reveals iron’s chemical behavior. It is a metal in the first. Iron Electrons Element.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image Iron Electrons Element The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron is a chemical element with symbol fe and atomic number 26. It’s a transition metal from group 8 of the periodic table’s first transition series. It is a metal in the first transition series. Fe, fe2+, and fe3+ electron. It is by mass. Iron Electrons Element.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron Electrons Element It is a metal in the first transition series. Iron is a chemical element with atomic number. It is by mass the most. Fe, fe2+, and fe3+ electron. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron can form various oxidation. It’s a transition metal from group 8 of. Iron Electrons Element.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Iron Electrons Element Iron is a chemical element with atomic number. It is a metal in the first transition series. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. This configuration reveals iron’s chemical behavior. It describes how electrons are distributed among the various atomic. Iron is a chemical element with symbol fe and atomic number. Iron Electrons Element.
From jaelynn-has-rodriguez.blogspot.com
Of the Following Which Element Has the Most Protons JaelynnhasRodriguez Iron Electrons Element The chemical element iron has the atomic number 26 and the symbol fe (from latin: It describes how electrons are distributed among the various atomic. It is a metal in the first transition series. It’s a transition metal from group 8 of the periodic table’s first transition series. Fe, fe2+, and fe3+ electron. Iron is a chemical element with atomic. Iron Electrons Element.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Electrons Element The electron configuration of iron is [ar] 3d6 4s2. Iron can form various oxidation. It describes how electrons are distributed among the various atomic. This configuration reveals iron’s chemical behavior. Iron is a chemical element with symbol fe and atomic number 26. Iron is a chemical element with atomic number. It’s a transition metal from group 8 of the periodic. Iron Electrons Element.
From www.vectorstock.com
Flashcard of iron with atomic mass Royalty Free Vector Image Iron Electrons Element Iron is a chemical element with atomic number. This configuration reveals iron’s chemical behavior. It is by mass the most. It describes how electrons are distributed among the various atomic. Fe, fe2+, and fe3+ electron. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. It’s a transition metal from group. Iron Electrons Element.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Electrons Element Iron can form various oxidation. This configuration reveals iron’s chemical behavior. It is by mass the most. Iron is a chemical element with symbol fe and atomic number 26. Fe, fe2+, and fe3+ electron. It’s a transition metal from group 8 of the periodic table’s first transition series. When we write the configuration we'll put all 26 electrons in orbitals. Iron Electrons Element.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Electrons Element Iron is a chemical element with atomic number. It’s a transition metal from group 8 of the periodic table’s first transition series. Iron is a chemical element with symbol fe and atomic number 26. It is a metal in the first transition series. Fe, fe2+, and fe3+ electron. It is by mass the most. The chemical element iron has the. Iron Electrons Element.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electrons Element The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It is a metal in the first transition series. Iron is a chemical element with symbol fe and atomic number 26. It is by mass the most. This configuration. Iron Electrons Element.
From cartoondealer.com
Iron Chemical Symbol As In The Periodic Table Stock Illustration Iron Electrons Element The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. It is a metal in the first transition series. This configuration reveals iron’s chemical behavior. The electron configuration of iron is [ar] 3d6 4s2. It’s a transition metal from group 8 of the periodic table’s first transition series. It is by mass the most.. Iron Electrons Element.
From www.alamy.com
Iron chemical element, Sign with atomic number and atomic weight Iron Electrons Element Fe, fe2+, and fe3+ electron. Iron can form various oxidation. It describes how electrons are distributed among the various atomic. The electron configuration of iron is [ar] 3d6 4s2. It is a metal in the first transition series. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. The electron configuration. Iron Electrons Element.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Electrons Element Iron can form various oxidation. Iron is a chemical element with symbol fe and atomic number 26. Fe, fe2+, and fe3+ electron. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. The electron configuration of iron is [ar] 3d6 4s2. It’s a transition metal from group 8 of the periodic table’s first transition. Iron Electrons Element.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron Electrons Element The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron can form various oxidation. Fe, fe2+, and fe3+ electron. It describes how electrons are distributed among the various atomic. Iron is a chemical element with atomic number. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. The. Iron Electrons Element.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electrons Element Iron is a chemical element with symbol fe and atomic number 26. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron can form various oxidation. Iron is a chemical element with atomic number. The chemical element iron has the atomic number 26 and the symbol fe (from latin: This configuration reveals iron’s. Iron Electrons Element.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Electrons Element The chemical element iron has the atomic number 26 and the symbol fe (from latin: The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron can form various oxidation. The electron configuration of iron is [ar] 3d6 4s2. Iron is a chemical element with symbol fe and atomic number 26. When we write. Iron Electrons Element.
From www.alamy.com
Structure of iron Stock Vector Images Alamy Iron Electrons Element It is by mass the most. Iron is a chemical element with symbol fe and atomic number 26. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It is a metal in the first transition series. The electron configuration of iron is [ar] 3d6 4s2. The electron configuration of iron refers to the arrangement. Iron Electrons Element.
From guides.byjusweb.com
Electronic Configuration of Iron Fe element Valency, Applications Iron Electrons Element The chemical element iron has the atomic number 26 and the symbol fe (from latin: When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron is a chemical element with atomic number. Iron can. Iron Electrons Element.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe Iron Electrons Element Fe, fe2+, and fe3+ electron. It is by mass the most. It describes how electrons are distributed among the various atomic. Iron can form various oxidation. It’s a transition metal from group 8 of the periodic table’s first transition series. It is a metal in the first transition series. When we write the configuration we'll put all 26 electrons in. Iron Electrons Element.
From modernizemodest1712.blogspot.com
40 orbital diagram for fe Diagram For You Iron Electrons Element Iron is a chemical element with atomic number. Fe, fe2+, and fe3+ electron. The electron configuration of iron is [ar] 3d6 4s2. Iron is a chemical element with symbol fe and atomic number 26. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. It is a metal in the first. Iron Electrons Element.
From www.animalia-life.club
Iron Atom Iron Electrons Element It describes how electrons are distributed among the various atomic. Iron is a chemical element with symbol fe and atomic number 26. This configuration reveals iron’s chemical behavior. It is by mass the most. It’s a transition metal from group 8 of the periodic table’s first transition series. Iron is a chemical element with atomic number. Iron can form various. Iron Electrons Element.
From www.youtube.com
A stepbystep description of how to write the electron configuration Iron Electrons Element It describes how electrons are distributed among the various atomic. Iron is a chemical element with symbol fe and atomic number 26. This configuration reveals iron’s chemical behavior. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Fe, fe2+, and fe3+ electron. Iron can form various oxidation. It’s a transition. Iron Electrons Element.
From www.istockphoto.com
70+ Iron Periodic Table Illustrations, RoyaltyFree Vector Graphics Iron Electrons Element The chemical element iron has the atomic number 26 and the symbol fe (from latin: The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. It is a metal in the first transition series. The electron configuration of iron is [ar] 3d6 4s2. It describes how electrons are distributed among the various atomic. This. Iron Electrons Element.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electrons Element Fe, fe2+, and fe3+ electron. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is a chemical element with atomic number. It is a metal in the first transition series. It is by. Iron Electrons Element.
From www.youtube.com
Valence Electrons for Fe (Iron) YouTube Iron Electrons Element This configuration reveals iron’s chemical behavior. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Fe, fe2+, and fe3+ electron. It’s a transition metal from group 8 of the periodic table’s first transition series. It describes how electrons are distributed among the various atomic. It is a metal in the first transition series.. Iron Electrons Element.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet Iron Electrons Element It’s a transition metal from group 8 of the periodic table’s first transition series. Iron can form various oxidation. It describes how electrons are distributed among the various atomic. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. This configuration reveals iron’s chemical behavior. The electron configuration of iron is. Iron Electrons Element.
From sciencenotes.org
List of Electron Configurations of Elements Iron Electrons Element Fe, fe2+, and fe3+ electron. Iron can form various oxidation. It describes how electrons are distributed among the various atomic. Iron is a chemical element with atomic number. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. It’s a transition metal from group 8 of the periodic table’s first transition series. When we. Iron Electrons Element.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Iron Electrons Element The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is a chemical element with atomic number. Iron is a chemical element with symbol fe and atomic number 26. Fe, fe2+, and fe3+ electron. It is a metal in the first transition series. Iron can form various oxidation. The electron configuration of iron is. Iron Electrons Element.