Bromine And Chlorine Reaction Equation . In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. The bromine reagent is in reddish color, and. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Activation of the electrophile by a lewis acid catalyst (or. These reactions are usually spontaneous. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Chlorine, bromine or iodine can be added to an alkene. Bromine + chlorine = bromine monochloride.
from www.chegg.com
Chlorine, bromine or iodine can be added to an alkene. The bromine reagent is in reddish color, and. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. Bromine + chlorine = bromine monochloride. These reactions are usually spontaneous. Activation of the electrophile by a lewis acid catalyst (or. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample.
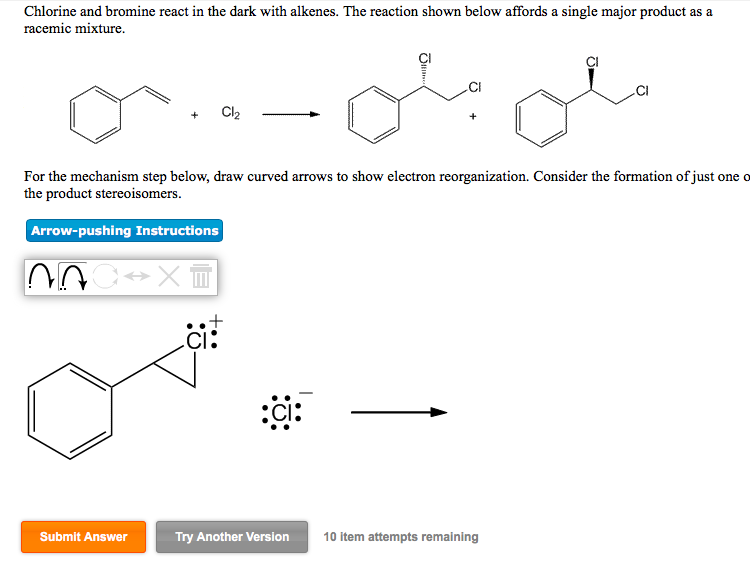
Solved Chlorine and bromine react in the dark with alkenes.
Bromine And Chlorine Reaction Equation The bromine reagent is in reddish color, and. Chlorine, bromine or iodine can be added to an alkene. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. The bromine reagent is in reddish color, and. These reactions are usually spontaneous. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine + chlorine = bromine monochloride. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Activation of the electrophile by a lewis acid catalyst (or. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps.
From www.numerade.com
SOLVEDBromine can be prepared by adding chlorine to an aqueous Bromine And Chlorine Reaction Equation Chlorine, bromine or iodine can be added to an alkene. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Br + cl =. Bromine And Chlorine Reaction Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine And Chlorine Reaction Equation If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Chlorine, bromine or iodine can be added to an alkene. The bromine reagent is in reddish color, and. Activation. Bromine And Chlorine Reaction Equation.
From www.dalalinstitute.com
Photochemical Reactions (HydrogenBromine & HydrogenChlorine Reactions Bromine And Chlorine Reaction Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine + chlorine = bromine monochloride. Activation of the electrophile by a lewis acid catalyst (or. The bromine reagent. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED Consider the reaction of bromide and chlorine Balance the Bromine And Chlorine Reaction Equation The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. These reactions are usually spontaneous. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. The bromine reagent is in reddish color, and. Br + cl = brcl is a synthesis. Bromine And Chlorine Reaction Equation.
From www.scribd.com
ATOPCV1 7 2 Photochemical Reactions Hydrogen Bromine and Hydrogen Bromine And Chlorine Reaction Equation These reactions are usually spontaneous. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. The bromine reagent is in reddish color, and. Draw the structure of. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVEDWrite equations to illustrate both aromatic and aliphatic Bromine And Chlorine Reaction Equation Activation of the electrophile by a lewis acid catalyst (or. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine reagent is in reddish color, and. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Bromine + chlorine = bromine monochloride. The. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED 1.Chlorine bromine and iodine all react with alkenes such as Bromine And Chlorine Reaction Equation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine reagent is in reddish color, and. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. These reactions are usually spontaneous. Draw the structure of the product formed when a given alkene undergoes. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED Chlorine and bromine react In the dark WIth alkenes The Bromine And Chlorine Reaction Equation The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Activation of the electrophile by a lewis acid catalyst (or. In. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED Chlorine can replace bromine in bromide compounds, forming Bromine And Chlorine Reaction Equation Bromine + chlorine = bromine monochloride. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Bromine And Chlorine Reaction Equation If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. The bromine reagent is in reddish color, and. Bromine + chlorine = bromine monochloride. Activation of the electrophile by a lewis acid catalyst (or. Alkenes react in the cold with pure liquid bromine,. Bromine And Chlorine Reaction Equation.
From gcse-revision-chemistry.blogspot.com
Beccy's Chemistry Revision 2018 Section 2 c) Summary Bromine And Chlorine Reaction Equation In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Activation of the electrophile by a lewis acid catalyst. Bromine And Chlorine Reaction Equation.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Bromine And Chlorine Reaction Equation The bromine reagent is in reddish color, and. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. Bromine + chlorine = bromine monochloride.. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine And Chlorine Reaction Equation If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an. Bromine And Chlorine Reaction Equation.
From solvedlib.com
Chlorine and bromine react in the dark with alkenes. … SolvedLib Bromine And Chlorine Reaction Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. The bromine reagent is in reddish color, and. Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Draw. Bromine And Chlorine Reaction Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine And Chlorine Reaction Equation Chlorine, bromine or iodine can be added to an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. These reactions are usually spontaneous. Activation of the electrophile by a lewis acid catalyst (or. Bromine + chlorine = bromine monochloride. Draw the structure of the product formed when. Bromine And Chlorine Reaction Equation.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Bromine And Chlorine Reaction Equation These reactions are usually spontaneous. Chlorine, bromine or iodine can be added to an alkene. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Bromine + chlorine = bromine monochloride. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample.. Bromine And Chlorine Reaction Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Bromine And Chlorine Reaction Equation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. These reactions are usually spontaneous. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Chlorine, bromine or iodine can be added to an. Bromine And Chlorine Reaction Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine And Chlorine Reaction Equation The bromine reagent is in reddish color, and. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Activation of the electrophile by a lewis acid catalyst (or. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Bromine + chlorine = bromine monochloride. These. Bromine And Chlorine Reaction Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine And Chlorine Reaction Equation The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Chlorine, bromine or iodine can be added to an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine reagent is. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVEDThe first preparation of chlorine was similar to the synthesis Bromine And Chlorine Reaction Equation Activation of the electrophile by a lewis acid catalyst (or. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. The bromine reagent is in reddish color, and. Chlorine, bromine or iodine can be added. Bromine And Chlorine Reaction Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine And Chlorine Reaction Equation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. These reactions are usually spontaneous. The bromine reagent is in reddish color, and. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Br + cl = brcl is a. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED Part A Enter a balanced equation for the reaction of chlorine Bromine And Chlorine Reaction Equation These reactions are usually spontaneous. The bromine reagent is in reddish color, and. Chlorine, bromine or iodine can be added to an alkene. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Bromine + chlorine = bromine monochloride. Activation of the electrophile by a lewis acid catalyst (or. In the. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are Bromine And Chlorine Reaction Equation Activation of the electrophile by a lewis acid catalyst (or. The bromine reagent is in reddish color, and. Bromine + chlorine = bromine monochloride. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. In the cases of chlorine, bromine, and iodine, electrophilic. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVEDThe nonspontaneous redox reaction of bromine and aqueous sodium Bromine And Chlorine Reaction Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. The bromine reagent is in reddish color, and. Bromine + chlorine = bromine monochloride. Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of. Bromine And Chlorine Reaction Equation.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Bromine And Chlorine Reaction Equation Activation of the electrophile by a lewis acid catalyst (or. Bromine + chlorine = bromine monochloride. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED What is the balanced chemical equation for bromine and chlorine Bromine And Chlorine Reaction Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Activation of the electrophile by a lewis acid catalyst (or. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The reaction between c=c double bond and bromine (br 2) can be used as a. Bromine And Chlorine Reaction Equation.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Bromine And Chlorine Reaction Equation The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like.. Bromine And Chlorine Reaction Equation.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Bromine And Chlorine Reaction Equation The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane.. Bromine And Chlorine Reaction Equation.
From byjus.com
36. fluorine react with water release oxygen but chlorine bromine Bromine And Chlorine Reaction Equation Activation of the electrophile by a lewis acid catalyst (or. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Draw the structure of. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVED 38. Write balanced chemical equation for the following reaction Bromine And Chlorine Reaction Equation In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. The bromine reagent is in reddish color, and. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. If you have a gaseous alkene like ethene, you can bubble it through. Bromine And Chlorine Reaction Equation.
From www.slideserve.com
PPT Q9 What are the chlorine and bromine reactions that destroy Bromine And Chlorine Reaction Equation Activation of the electrophile by a lewis acid catalyst (or. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. These reactions are usually spontaneous. The bromine reagent is. Bromine And Chlorine Reaction Equation.
From slideplayer.com
Chemical Equations Chapter ppt download Bromine And Chlorine Reaction Equation Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Activation of the electrophile by a lewis acid catalyst (or. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. In the cases of. Bromine And Chlorine Reaction Equation.
From www.youtube.com
How to Balance KBr + Cl2 = KCl + Br2 (Potassium bromide + Chlorine gas Bromine And Chlorine Reaction Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like. The bromine reagent is in reddish color, and. Bromine + chlorine = bromine monochloride. Activation of the. Bromine And Chlorine Reaction Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Bromine And Chlorine Reaction Equation Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Bromine + chlorine = bromine monochloride. These reactions are usually spontaneous. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. If you have a gaseous. Bromine And Chlorine Reaction Equation.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Bromine And Chlorine Reaction Equation In the cases of chlorine, bromine, and iodine, electrophilic aromatic substitution follows three steps. Bromine + chlorine = bromine monochloride. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine And Chlorine Reaction Equation.