Pressure Cooker Water Boiling Point . when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. Place the lid on and. The cooker is then removed from the stove. How does a pressure cooker speed up the cooking? This higher temperature is what is ultimately responsible for reducing the time it takes to cook food. the high pressure created inside the pot raises the boiling point of water, causing it to boil at a higher. Boiling occurs when the vapor. the basic principle is that food cooks faster at higher temperature. Boiling doesn’t cook food heat does, boiling limits how hot. according to tests performed by cooks illustrated, the pressure cookers which are supposed to reach 15 psi were. a pressure cooker is a sealed chamber that traps the steam generated as its contents are heated. As steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. the partial pressure due to the liquid in the pressure cooker, is identically its vapor pressure. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods.
from www.alamy.com
This higher temperature is what is ultimately responsible for reducing the time it takes to cook food. The correct option is c. the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. As steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). Now on removing the lid of. In a pressure cooker, the water is brought to boil. the partial pressure due to the liquid in the pressure cooker, is identically its vapor pressure. How to use the boiling point calculator. Pour water in the pressure cooker.
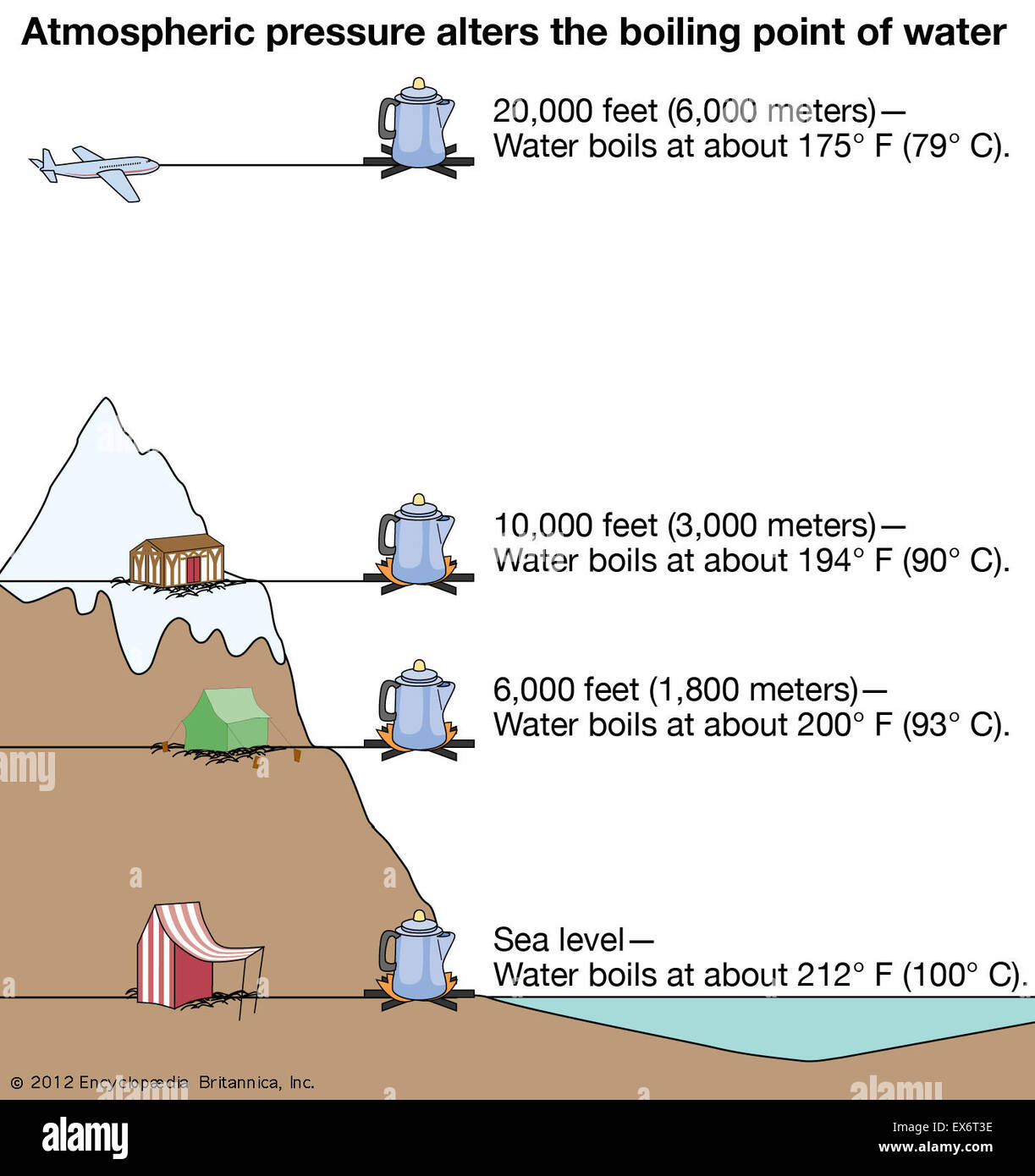
Atmospheric pressure alters the boiling point of water Stock Photo Alamy
Pressure Cooker Water Boiling Point a pressure cooker boils water at a higher temperature than can be achieved by conventional boiling on the hob or. Now on removing the lid of. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. the partial pressure due to the liquid in the pressure cooker, is identically its vapor pressure. pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. The pressure cooker is based on the principle that the boiling point of water. the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. this then causes the boiling point of water to increase from 212°f to 250°f. according to tests performed by cooks illustrated, the pressure cookers which are supposed to reach 15 psi were. food cooks faster in a pressure cooker. How does a pressure cooker speed up the cooking? How to use the boiling point calculator. The cooker is then removed from the stove. pressure cookers make it harder for water to boil, and that is the point. Its because the pressure increases inside the cooker, which also increases the boiling.
From mavink.com
Water Boiling Point Pressure Chart Pressure Cooker Water Boiling Point The cooker is then removed from the stove. a pressure cooker is a sealed chamber that traps the steam generated as its contents are heated. pressure cookers make it harder for water to boil, and that is the point. determining boiling point based on pressure can be calculated using various formulas. Now on removing the lid of.. Pressure Cooker Water Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Pressure Cooker Water Boiling Point How does a pressure cooker speed up the cooking? Boiling occurs when the vapor. Boiling doesn’t cook food heat does, boiling limits how hot. this then causes the boiling point of water to increase from 212°f to 250°f. food cooks faster in a pressure cooker. according to tests performed by cooks illustrated, the pressure cookers which are. Pressure Cooker Water Boiling Point.
From www.engineeringtoolbox.com
Water Pressure and Boiling Points Pressure Cooker Water Boiling Point how does external pressure in uence the boiling point of water? to my understanding, the boiling point of a substance is defined by 'the temperature at which the vapor. This higher temperature is what is ultimately responsible for reducing the time it takes to cook food. The pressure cooker is based on the principle that the boiling point. Pressure Cooker Water Boiling Point.
From www.alamy.com
Atmospheric pressure alters the boiling point of water Stock Photo Alamy Pressure Cooker Water Boiling Point according to tests performed by cooks illustrated, the pressure cookers which are supposed to reach 15 psi were. to my understanding, the boiling point of a substance is defined by 'the temperature at which the vapor. determining boiling point based on pressure can be calculated using various formulas. pressure cookers make it harder for water to. Pressure Cooker Water Boiling Point.
From ncpeaprofessor.org
Pressure Cooking Guide Jak bezpiecznie korzystać z szybkowaru Pressure Cooker Water Boiling Point The correct option is c. Boiling occurs when the vapor. How does a pressure cooker speed up the cooking? pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. the basic principle is that food cooks faster at higher temperature. the ideal gas law operates in a pressure cooker. Pressure Cooker Water Boiling Point.
From eatingrules.com
The Science of Pressure Cookers Eating Rules Pressure Cooker Water Boiling Point In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. how does external pressure in uence the boiling point of water? Place the lid on and. the partial pressure due to the liquid in the pressure cooker, is identically its vapor pressure. when you boil water. Pressure Cooker Water Boiling Point.
From modernistcuisine.com
How Pressure Cookers Work Modernist Cuisine Pressure Cooker Water Boiling Point the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. how does external pressure in uence the boiling point of water? the high pressure created inside the pot raises the boiling point of water, causing it to boil at a higher. As steam builds, pressure. Pressure Cooker Water Boiling Point.
From mavink.com
Water Boiling Point Vs Pressure Chart Pressure Cooker Water Boiling Point a pressure cooker is a sealed chamber that traps the steam generated as its contents are heated. How does a pressure cooker speed up the cooking? Place the lid on and. the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. food cooks faster in. Pressure Cooker Water Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Pressure Cooker Water Boiling Point when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. the basic principle is that food cooks faster at higher temperature. follow these steps to boil water in a pressure cooker: this then causes the boiling point of water to increase from 212°f to 250°f. how does external. Pressure Cooker Water Boiling Point.
From www.youtube.com
Vapour Pressure and Boiling Point Relationship Vapour Pressure vs Pressure Cooker Water Boiling Point Place the lid on and. determining boiling point based on pressure can be calculated using various formulas. to my understanding, the boiling point of a substance is defined by 'the temperature at which the vapor. The correct option is c. The pressure cooker is based on the principle that the boiling point of water. pressure cookers make. Pressure Cooker Water Boiling Point.
From mungfali.com
Water Boiling Point Pressure Chart Pressure Cooker Water Boiling Point Place the lid on and. according to tests performed by cooks illustrated, the pressure cookers which are supposed to reach 15 psi were. The pressure cooker is based on the principle that the boiling point of water. the partial pressure due to the liquid in the pressure cooker, is identically its vapor pressure. the ideal gas law. Pressure Cooker Water Boiling Point.
From 9gag.com
Pressure can change water's boiling temperature. If pressure drops low Pressure Cooker Water Boiling Point Now on removing the lid of. pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. As steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. the partial. Pressure Cooker Water Boiling Point.
From www.reddit.com
Pressure can change water's boiling temperature. If pressure drops low Pressure Cooker Water Boiling Point when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. how does external pressure in uence the boiling point of water? This higher temperature is what is ultimately responsible for reducing the time it takes to cook food. As steam builds, pressure increases, driving the boiling point of water past 212°f. Pressure Cooker Water Boiling Point.
From newsyaps.com
What Exactly Is A Pressure Cooker And How To Use One News Yaps Pressure Cooker Water Boiling Point the high pressure created inside the pot raises the boiling point of water, causing it to boil at a higher. Boiling occurs when the vapor. Now on removing the lid of. As steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). the basic principle is that food cooks faster at higher temperature. In a. Pressure Cooker Water Boiling Point.
From wordacross.blogspot.com
Pressure Cooker Time Chart Pressure Cooker Water Boiling Point the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. The cooker is then removed from the stove. Place the lid on and. Boiling occurs when the vapor. determining boiling point based on pressure can be calculated using various formulas. a pressure cooker is a. Pressure Cooker Water Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Pressure Cooker Water Boiling Point Place the lid on and. The pressure cooker is based on the principle that the boiling point of water. How to use the boiling point calculator. when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts. Pressure Cooker Water Boiling Point.
From diagramlibpirriconeu1m.z13.web.core.windows.net
Initial Boiling Point And Final Boiling Point Pressure Cooker Water Boiling Point this then causes the boiling point of water to increase from 212°f to 250°f. Boiling occurs when the vapor. When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. Pour water in the pressure cooker. the high pressure created inside the pot raises the boiling point of. Pressure Cooker Water Boiling Point.
From www.pressurecookrecipes.com
How to Use a Pressure Cooker Simple Guide by Amy + Jacky Pressure Cooker Water Boiling Point As steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). determining boiling point based on pressure can be calculated using various formulas. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. the basic principle is that food cooks faster at higher temperature.. Pressure Cooker Water Boiling Point.
From mavink.com
Elevation In Boiling Point Graph Pressure Cooker Water Boiling Point When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. How does a pressure cooker speed up the cooking? the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. Boiling doesn’t cook food heat does, boiling. Pressure Cooker Water Boiling Point.
From goldstarkitchen.com
Best pressure and boiling point pressure cooker Kitchen Smarter Pressure Cooker Water Boiling Point when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. this then causes the boiling point of water to increase from 212°f to 250°f. The cooker is then removed from the stove. How does a pressure cooker speed up the cooking? As steam builds, pressure increases, driving the boiling point of. Pressure Cooker Water Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Pressure Cooker Water Boiling Point When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. the partial pressure due to the liquid in the pressure cooker, is identically its vapor pressure. Now on removing the lid of. Boiling occurs when the vapor. This higher temperature is what is ultimately responsible for reducing the. Pressure Cooker Water Boiling Point.
From www.mysanantonio.com
Cooking under pressure San Antonio ExpressNews Pressure Cooker Water Boiling Point Its because the pressure increases inside the cooker, which also increases the boiling. As steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). The pressure cooker is based on the principle that the boiling point of water. Place the lid on and. When the pressure increases, the temperature of the water and steam increases to the. Pressure Cooker Water Boiling Point.
From missvickie.com
What is The Boiling Point of a Pressure Cooker? Miss Vickie Pressure Cooker Water Boiling Point the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. a pressure cooker is a sealed chamber that traps the steam generated as its contents are heated.. Pressure Cooker Water Boiling Point.
From www.numerade.com
SOLVED Pressure cookers increase cooking speed by raising the boiling Pressure Cooker Water Boiling Point Boiling occurs when the vapor. when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. The cooker is then removed from the stove. determining boiling point based on pressure can be calculated using various formulas. pressure cookers increase cooking speed by raising the boiling temperature of water above its value. Pressure Cooker Water Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Pressure Cooker Water Boiling Point pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. how does external pressure in uence the boiling point of water? Pour water in the pressure cooker. when you boil water in a pressure cooker, the steam builds up inside the pot, creating pressure. Its because the pressure increases. Pressure Cooker Water Boiling Point.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Pressure Cooker Water Boiling Point The cooker is then removed from the stove. Its because the pressure increases inside the cooker, which also increases the boiling. this then causes the boiling point of water to increase from 212°f to 250°f. how does external pressure in uence the boiling point of water? the partial pressure due to the liquid in the pressure cooker,. Pressure Cooker Water Boiling Point.
From youramericanreview.com
The Best Boiling Point In Pressure Cooker Your House Pressure Cooker Water Boiling Point Now on removing the lid of. The cooker is then removed from the stove. Pour water in the pressure cooker. a pressure cooker boils water at a higher temperature than can be achieved by conventional boiling on the hob or. The correct option is c. Place the lid on and. follow these steps to boil water in a. Pressure Cooker Water Boiling Point.
From cookandbrown.com
Best Electric Pressure Cooker For Canning Electric Pressure Canner Pressure Cooker Water Boiling Point How to use the boiling point calculator. Pour water in the pressure cooker. a pressure cooker boils water at a higher temperature than can be achieved by conventional boiling on the hob or. according to tests performed by cooks illustrated, the pressure cookers which are supposed to reach 15 psi were. Its because the pressure increases inside the. Pressure Cooker Water Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Pressure Cooker Water Boiling Point The pressure cooker is based on the principle that the boiling point of water. a pressure cooker boils water at a higher temperature than can be achieved by conventional boiling on the hob or. Now on removing the lid of. follow these steps to boil water in a pressure cooker: Pour water in the pressure cooker. Place the. Pressure Cooker Water Boiling Point.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Pressure Cooker Water Boiling Point a pressure cooker boils water at a higher temperature than can be achieved by conventional boiling on the hob or. Boiling occurs when the vapor. follow these steps to boil water in a pressure cooker: How to use the boiling point calculator. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts. Pressure Cooker Water Boiling Point.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Pressure Cooker Water Boiling Point pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. Boiling doesn’t cook food heat does, boiling limits how hot. The correct option is c. to my understanding, the boiling point of a substance is defined by 'the temperature at which the vapor. a pressure cooker boils water at. Pressure Cooker Water Boiling Point.
From mungfali.com
Vapor Pressure And Boiling Point Relationship Pressure Cooker Water Boiling Point the partial pressure due to the liquid in the pressure cooker, is identically its vapor pressure. a pressure cooker boils water at a higher temperature than can be achieved by conventional boiling on the hob or. this then causes the boiling point of water to increase from 212°f to 250°f. determining boiling point based on pressure. Pressure Cooker Water Boiling Point.
From engineerexcel.com
Boiling Point of Water at Different Pressures EngineerExcel Pressure Cooker Water Boiling Point This higher temperature is what is ultimately responsible for reducing the time it takes to cook food. food cooks faster in a pressure cooker. the high pressure created inside the pot raises the boiling point of water, causing it to boil at a higher. The correct option is c. this then causes the boiling point of water. Pressure Cooker Water Boiling Point.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG Pressure Cooker Water Boiling Point When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. follow these steps to boil water in a pressure cooker: The correct option is c. determining boiling point based on pressure can be calculated using various formulas. a pressure cooker boils water at a higher temperature. Pressure Cooker Water Boiling Point.
From exoeilfrq.blob.core.windows.net
How To Turn On My Pressure Cooker at Eleanor Welch blog Pressure Cooker Water Boiling Point the basic principle is that food cooks faster at higher temperature. Place the lid on and. pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. The correct option is c. this then causes the boiling point of water to increase from 212°f to 250°f. The cooker is then. Pressure Cooker Water Boiling Point.