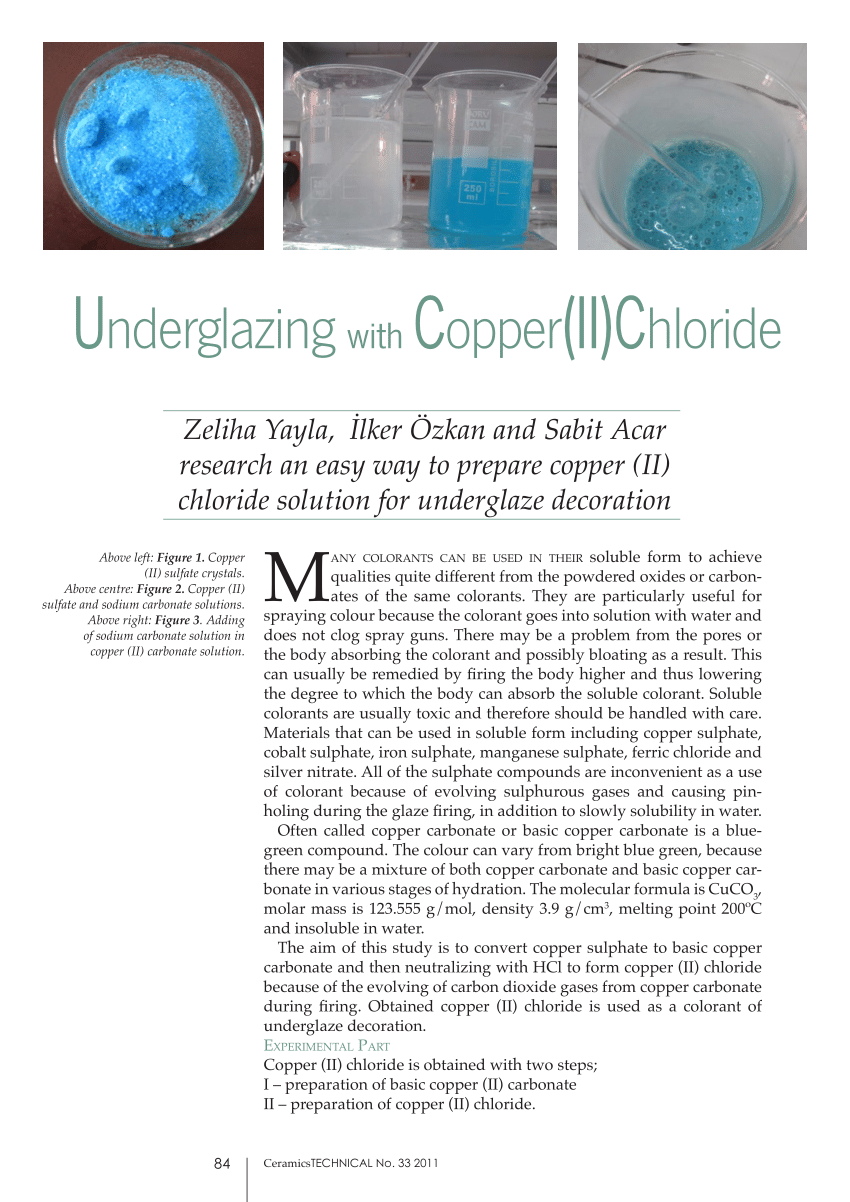
Copper Ii Chloride Dissolved In Water . Refer to the chart below to find reference values. — copper (ii) chloride formula. water temperature can have a significant effect on the solubility of compounds. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride’s formula is cucl2. So for each mole of copper chloride, full dissociation will give you. Copper (ii) chloride consists of one copper. It is a brown solid. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — in this video we will describe the equation cucl2 + h2o and write what. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine.
from www.researchgate.net
— in this video we will describe the equation cucl2 + h2o and write what. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride’s formula is cucl2. water temperature can have a significant effect on the solubility of compounds. — copper (ii) chloride formula. So for each mole of copper chloride, full dissociation will give you. Copper (ii) chloride consists of one copper. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine.
(PDF) Underglazing with copper(II)chloride
Copper Ii Chloride Dissolved In Water — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. — in this video we will describe the equation cucl2 + h2o and write what. water temperature can have a significant effect on the solubility of compounds. Copper (ii) chloride’s formula is cucl2. — copper (ii) chloride formula. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Refer to the chart below to find reference values. It is a brown solid. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. So for each mole of copper chloride, full dissociation will give you. Copper (ii) chloride consists of one copper. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol.
From www.researchgate.net
UVvis spectra of copper(II) chloride dissolved in six different Copper Ii Chloride Dissolved In Water It is a brown solid. Copper (ii) chloride consists of one copper. water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values. Copper (ii) chloride’s formula is cucl2. So for each mole of copper chloride, full dissociation will give you. copper (ii) chloride, also known as cupric. Copper Ii Chloride Dissolved In Water.
From 2012books.lardbucket.org
Describing Electrochemical Cells Copper Ii Chloride Dissolved In Water water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Copper (ii) chloride consists of one copper. It is a brown solid. — copper(ii) chloride, also known as cupric chloride, is an. Copper Ii Chloride Dissolved In Water.
From www.animalia-life.club
Copper Chloride Copper Ii Chloride Dissolved In Water — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. — in this video we will describe the equation cucl2 + h2o and write what. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. copper (ii) chloride, also known as. Copper Ii Chloride Dissolved In Water.
From www.chemkits.eu
Copper(II) chloride dihydrate, 99.5+, 10125130 Copper Ii Chloride Dissolved In Water copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — in this video we will describe the equation cucl2 + h2o and write what. — copper (ii) chloride formula. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. It is a. Copper Ii Chloride Dissolved In Water.
From www.youtube.com
Aluminum and Copper (II) Chloride Reaction YouTube Copper Ii Chloride Dissolved In Water — copper (ii) chloride formula. Copper (ii) chloride consists of one copper. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Refer to the chart below to find reference values. Copper (ii) chloride’s formula is cucl2. — in this video we will describe the equation. Copper Ii Chloride Dissolved In Water.
From www.coursehero.com
[Solved] Please see below question V The names and chemical formulae of Copper Ii Chloride Dissolved In Water It is a brown solid. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. water temperature can have. Copper Ii Chloride Dissolved In Water.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Chloride Copper Ii Chloride Dissolved In Water Copper (ii) chloride consists of one copper. Copper (ii) chloride’s formula is cucl2. So for each mole of copper chloride, full dissociation will give you. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Refer to the chart below to find reference values. It is a brown. Copper Ii Chloride Dissolved In Water.
From www.youtube.com
How to separate sand and copper(II) sulfate salt from its mixture Copper Ii Chloride Dissolved In Water water temperature can have a significant effect on the solubility of compounds. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. So for each mole of copper chloride, full dissociation will give you. — in this video we will describe the equation cucl2 + h2o and write what. — copper(ii) chloride, also known. Copper Ii Chloride Dissolved In Water.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog Copper Ii Chloride Dissolved In Water Refer to the chart below to find reference values. water temperature can have a significant effect on the solubility of compounds. Copper (ii) chloride consists of one copper. — copper (ii) chloride formula. — in this video we will describe the equation cucl2 + h2o and write what. Copper (ii) chloride’s formula is cucl2. — if. Copper Ii Chloride Dissolved In Water.
From digital-analysis.com
Heavy Metal Reduction for Industrial Wastewater Copper Ii Chloride Dissolved In Water water temperature can have a significant effect on the solubility of compounds. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride’s. Copper Ii Chloride Dissolved In Water.
From www.coursehero.com
[Solved] During part B of the lab, the copper chloride anhydrate was Copper Ii Chloride Dissolved In Water Copper (ii) chloride consists of one copper. water temperature can have a significant effect on the solubility of compounds. So for each mole of copper chloride, full dissociation will give you. Copper (ii) chloride’s formula is cucl2. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — if you assume a molecule $\ce{cucl2}$ there. Copper Ii Chloride Dissolved In Water.
From chemicaldb.netlify.app
Copper 2 sulfate with water heat Copper Ii Chloride Dissolved In Water Copper (ii) chloride’s formula is cucl2. Refer to the chart below to find reference values. water temperature can have a significant effect on the solubility of compounds. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — if you assume a molecule $\ce{cucl2}$ there is. Copper Ii Chloride Dissolved In Water.
From www.echemi.com
How can you find the concentration of copper(II) ions in a solution of Copper Ii Chloride Dissolved In Water So for each mole of copper chloride, full dissociation will give you. Copper (ii) chloride consists of one copper. — in this video we will describe the equation cucl2 + h2o and write what. Copper (ii) chloride’s formula is cucl2. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. — copper(ii) chloride,. Copper Ii Chloride Dissolved In Water.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry Copper Ii Chloride Dissolved In Water It is a brown solid. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — copper (ii) chloride formula. Refer to the chart below. Copper Ii Chloride Dissolved In Water.
From slideplayer.com
Molarity (m). ppt download Copper Ii Chloride Dissolved In Water The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. It is a brown solid. — copper (ii) chloride formula. Refer to the chart below to find reference values. So for each mole of copper chloride, full dissociation will give you. water temperature can have a significant effect on the solubility of compounds. copper. Copper Ii Chloride Dissolved In Water.
From dxouounyb.blob.core.windows.net
Mixing Aluminum With Copper(Ii) Chloride Temperature at Valerie Ohler blog Copper Ii Chloride Dissolved In Water Copper (ii) chloride consists of one copper. Refer to the chart below to find reference values. — copper (ii) chloride formula. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and. Copper Ii Chloride Dissolved In Water.
From www.sarchemlabs.com
What is Copper II Chloride Applications, FAQs & More Copper Ii Chloride Dissolved In Water — in this video we will describe the equation cucl2 + h2o and write what. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride consists of one copper. So for each mole of copper chloride, full dissociation will give you. — copper(ii). Copper Ii Chloride Dissolved In Water.
From www.youtube.com
Electrolysis of Copper Chloride solution National 5 YouTube Copper Ii Chloride Dissolved In Water The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — copper (ii) chloride formula. It is a brown solid. Copper (ii) chloride’s formula is cucl2. So for each mole of copper chloride, full dissociation will give you. water temperature can have a significant effect on the solubility of compounds. Copper (ii) chloride consists of. Copper Ii Chloride Dissolved In Water.
From exohpqwmj.blob.core.windows.net
Which Crystals Dissolve In Water at Vivian Lamotte blog Copper Ii Chloride Dissolved In Water Copper (ii) chloride consists of one copper. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Copper (ii) chloride’s formula is cucl2. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. It is a brown solid. — in this video. Copper Ii Chloride Dissolved In Water.
From www.reddit.com
Why is the answer D and not C? Copper(II) sulfate is soluble in water Copper Ii Chloride Dissolved In Water — copper (ii) chloride formula. So for each mole of copper chloride, full dissociation will give you. Copper (ii) chloride’s formula is cucl2. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. It is a brown solid. Copper (ii) chloride consists of one copper. —. Copper Ii Chloride Dissolved In Water.
From testbook.com
Copper (II) Chloride Formula Structure, Properties and Uses Copper Ii Chloride Dissolved In Water It is a brown solid. — copper (ii) chloride formula. — in this video we will describe the equation cucl2 + h2o and write what. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. So for each mole of copper chloride, full dissociation will give you. copper (ii) chloride, also known as cupric. Copper Ii Chloride Dissolved In Water.
From www.chegg.com
Solved Mole Ratios Copper(II) chloride (CuCl,; 0.98 g) was Copper Ii Chloride Dissolved In Water — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. water temperature can have a significant effect on the solubility of compounds. Copper (ii) chloride’s. Copper Ii Chloride Dissolved In Water.
From www.youtube.com
dissolving copper chloride in distilled water YouTube Copper Ii Chloride Dissolved In Water It is a brown solid. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Copper (ii) chloride’s formula is cucl2. — copper (ii) chloride formula. The exact mass and the monoisotopic. Copper Ii Chloride Dissolved In Water.
From jordutah.weebly.com
Copper ii chloride jordutah Copper Ii Chloride Dissolved In Water — copper (ii) chloride formula. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. The exact mass and the monoisotopic mass of cupric chloride. Copper Ii Chloride Dissolved In Water.
From www.chegg.com
Solved The salt copper(II) chloride dissolves in water Copper Ii Chloride Dissolved In Water copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — in this video we will describe the equation cucl2 + h2o and write what. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — copper(ii) chloride, also known as cupric chloride,. Copper Ii Chloride Dissolved In Water.
From www.pw.live
Copper I Chloride Formula, Structure, Properties, Uses Copper Ii Chloride Dissolved In Water Refer to the chart below to find reference values. copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride consists of one copper.. Copper Ii Chloride Dissolved In Water.
From www.youtube.com
Copper (II) Sulfate + Sodium Carbonate = (Double Displacement with Copper Ii Chloride Dissolved In Water — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride consists of one copper. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — in this video we will describe the equation cucl2 + h2o and write what. It is. Copper Ii Chloride Dissolved In Water.
From www.animalia-life.club
Copper Chloride Copper Ii Chloride Dissolved In Water Copper (ii) chloride’s formula is cucl2. Copper (ii) chloride consists of one copper. — in this video we will describe the equation cucl2 + h2o and write what. Refer to the chart below to find reference values. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2.. Copper Ii Chloride Dissolved In Water.
From www.sciencephoto.com
Copper(II) Chloride Stock Image C027/9485 Science Photo Library Copper Ii Chloride Dissolved In Water — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Refer to the chart below to find reference values. Copper (ii) chloride’s formula is cucl2. Copper (ii) chloride consists of one copper. So for each mole of copper chloride, full dissociation will give you. The exact mass and. Copper Ii Chloride Dissolved In Water.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Ii Chloride Dissolved In Water The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Refer to the chart below to find reference values. — in this video we will describe the equation cucl2 + h2o and write what. — copper (ii) chloride formula. Copper. Copper Ii Chloride Dissolved In Water.
From www.researchgate.net
(PDF) Underglazing with copper(II)chloride Copper Ii Chloride Dissolved In Water Copper (ii) chloride’s formula is cucl2. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. — in this video we will describe the equation cucl2 + h2o and write what. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. So for each mole of copper chloride, full dissociation will. Copper Ii Chloride Dissolved In Water.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Chloride Copper Ii Chloride Dissolved In Water So for each mole of copper chloride, full dissociation will give you. Refer to the chart below to find reference values. It is a brown solid. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. The exact mass and the monoisotopic mass of cupric chloride is 132.867. Copper Ii Chloride Dissolved In Water.
From maverickkruwfowler.blogspot.com
Colour of Copper Chloride MaverickkruwFowler Copper Ii Chloride Dissolved In Water — copper (ii) chloride formula. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. — copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. — in this video we will describe the equation cucl2 + h2o and write what. . Copper Ii Chloride Dissolved In Water.
From www.numerade.com
SOLVED A solution is prepared by dissolving 63.8 grams of copper (II Copper Ii Chloride Dissolved In Water Refer to the chart below to find reference values. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. — copper (ii) chloride formula. water temperature can have a significant effect on the solubility of compounds. copper (ii) chloride,. Copper Ii Chloride Dissolved In Water.
From www.flinnsci.com
Copper(II) Chloride, Reagent, 500 g Flinn Scientific Copper Ii Chloride Dissolved In Water — copper (ii) chloride formula. Copper (ii) chloride consists of one copper. — if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. water temperature can have a significant effect on the solubility of compounds. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. It is a brown solid. Refer. Copper Ii Chloride Dissolved In Water.