What Three Liquids Don T Mix . Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. These three liquids do not mix easily, so when you shake. Two liquids that do not really mix well together, such as oil and water, are described as immiscible. They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). Two liquids that appear to mix completely together are said to be miscible. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil and water don't mix. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible.
from www.doubtnut.com
Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. Two liquids that appear to mix completely together are said to be miscible. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. These three liquids do not mix easily, so when you shake. When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil and water don't mix. Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12).
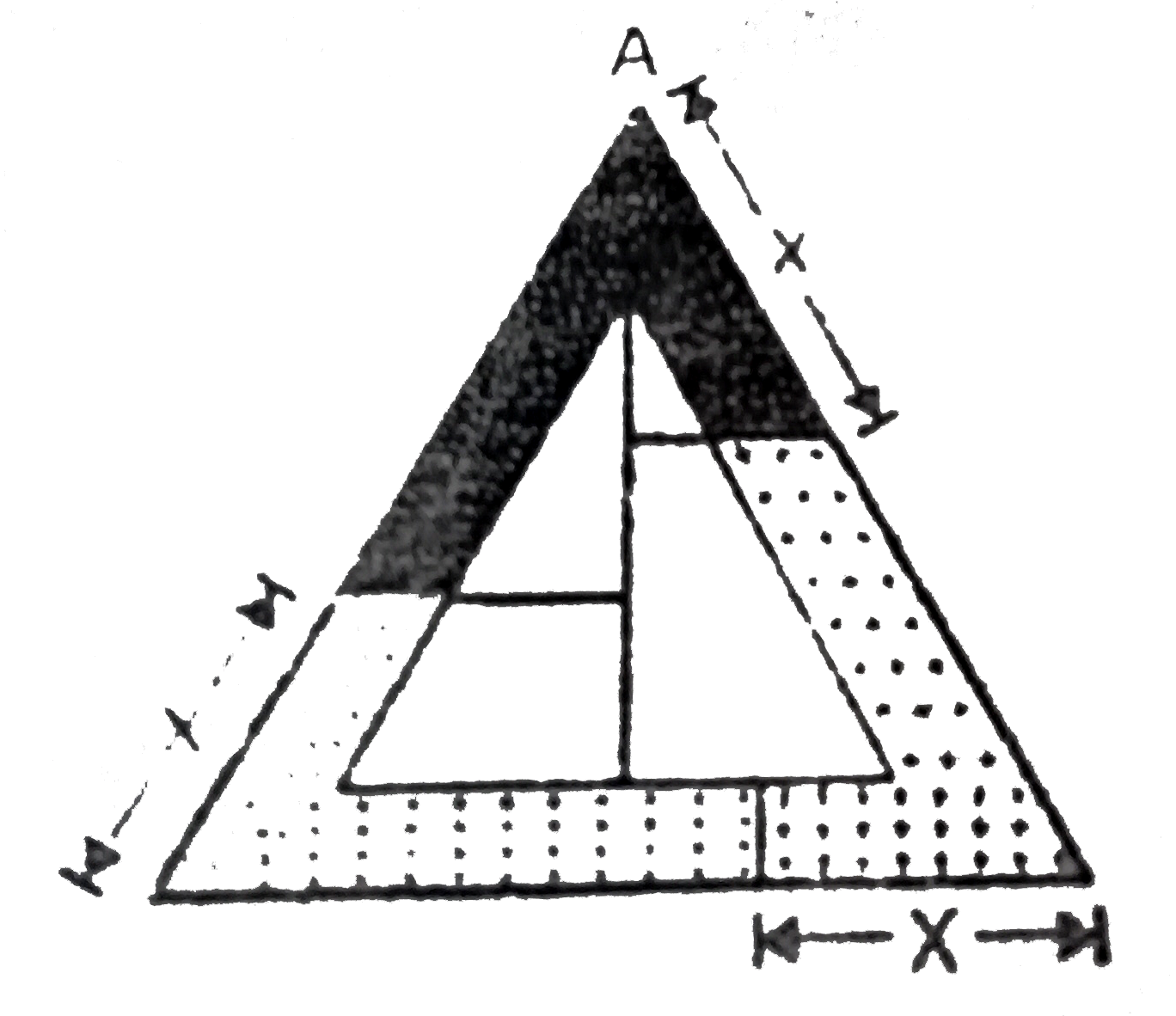
A closed tube in the form of an equilateral triangle of side l contain
What Three Liquids Don T Mix They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil and water don't mix. These three liquids do not mix easily, so when you shake. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. Two liquids that appear to mix completely together are said to be miscible. They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). Two liquids that do not really mix well together, such as oil and water, are described as immiscible.
From www.dreamstime.com
OIL and WATER. School Experiment Stock Vector Illustration of What Three Liquids Don T Mix These three liquids do not mix easily, so when you shake. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. Two liquids that do not really mix well together, such as oil and water, are described as immiscible. Toluene is a common solvent in paint thinners and other industrial chemicals, and these. What Three Liquids Don T Mix.
From www.labroots.com
Check Out This Density Experiment Videos What Three Liquids Don T Mix Two liquids that do not really mix well together, such as oil and water, are described as immiscible. These three liquids do not mix easily, so when you shake. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12).. What Three Liquids Don T Mix.
From www.toppr.com
A closed tube in the form of an equilateral triangle of side ℓ contains What Three Liquids Don T Mix Two liquids that appear to mix completely together are said to be miscible. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is. What Three Liquids Don T Mix.
From www.doubtnut.com
A closed tube in the form of an equilateral triangle of side l contain What Three Liquids Don T Mix They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. These three liquids do not mix easily, so when you shake. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. The components are said to be immiscible. in contrast, fluids that do mix together. What Three Liquids Don T Mix.
From www.generationcontracting.com
Are You Disinfecting Safely? Generation Contracting What Three Liquids Don T Mix They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Two liquids that appear to mix completely together are said to be miscible. Two liquids that do not really mix well together, such as oil and water, are described as immiscible. Toluene is a common solvent in paint thinners and other industrial. What Three Liquids Don T Mix.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID2143168 What Three Liquids Don T Mix Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. Two liquids that appear to mix completely together are said to be miscible. Liquids tend to be immiscible when the force of attraction between the molecules. What Three Liquids Don T Mix.
From www.pinterest.com
20 Common Household Cleaning Products You Should Never Mix Cleaning What Three Liquids Don T Mix Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12).. What Three Liquids Don T Mix.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize What Three Liquids Don T Mix Two liquids that appear to mix completely together are said to be miscible. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. Liquids tend to be immiscible when the force of. What Three Liquids Don T Mix.
From sciencenotes.org
Liquid Definition Examples of Liquids What Three Liquids Don T Mix Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Two liquids that appear to mix completely together are said to be miscible. Two liquids that do not really mix well together, such as oil and water, are described as immiscible. When two liquids combine to form a solution,. What Three Liquids Don T Mix.
From www.instructables.com
NonMixing Liquids 6 Steps (with Pictures) Instructables What Three Liquids Don T Mix Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. The components are said to be immiscible. in. What Three Liquids Don T Mix.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID2061355 What Three Liquids Don T Mix Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. These three liquids do not mix easily, so when you shake. Two liquids that do not really mix well together, such as oil and water, are. What Three Liquids Don T Mix.
From quizlet.com
Combining and Separating Flashcards Quizlet What Three Liquids Don T Mix Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. From least dense to. What Three Liquids Don T Mix.
From www.alamy.com
An illustration of immiscible liquids water and oil in an emulsion What Three Liquids Don T Mix They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. When two liquids combine to form a solution, they are called miscible. if they can't be. What Three Liquids Don T Mix.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The What Three Liquids Don T Mix Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Two liquids that do not really mix well together, such as oil and water, are described as immiscible. From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. When two liquids combine to. What Three Liquids Don T Mix.
From sciencenotes.org
Household Chemicals You Should Never Mix What Three Liquids Don T Mix Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. Two liquids that do not really mix well together, such as oil and water, are described as immiscible. The components are said to be immiscible. in. What Three Liquids Don T Mix.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID What Three Liquids Don T Mix Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). These three liquids do not mix easily, so when you shake. Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Two liquids that do not really mix well together, such as oil and water, are described as immiscible.. What Three Liquids Don T Mix.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning What Three Liquids Don T Mix They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Two liquids that appear to mix completely together are said to be miscible. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. Toluene is. What Three Liquids Don T Mix.
From www.pinterest.com
Delicious Density Experiment 🍇age 3 🍇 Here’s a fun and yummy way to What Three Liquids Don T Mix When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil and water don't mix. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and. What Three Liquids Don T Mix.
From www.pinterest.com
dont mix liquids and solids Healthdirectapp Liquid, Meals, Food What Three Liquids Don T Mix Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. Immiscibility is the property where two substances are not capable of combining to form. What Three Liquids Don T Mix.
From slideplayer.com
Solutions Chapter ppt download What Three Liquids Don T Mix They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). Two liquids that. What Three Liquids Don T Mix.
From www.pinterest.com
100 best 3 States of matter images on Pinterest Teaching science What Three Liquids Don T Mix Two liquids that appear to mix completely together are said to be miscible. Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. These three liquids do not mix easily, so when you shake. Immiscibility is the property where two substances. What Three Liquids Don T Mix.
From www.youtube.com
DON'T MIX THESE LIQUIDS The Chemist Gameplay New Chemist Simulator What Three Liquids Don T Mix These three liquids do not mix easily, so when you shake. Two liquids that do not really mix well together, such as oil and water, are described as immiscible. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and. What Three Liquids Don T Mix.
From www.slideshare.net
What can we observe about liquids What Three Liquids Don T Mix Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil and. What Three Liquids Don T Mix.
From www.thoughtco.com
Mixture Definition and Examples in Science What Three Liquids Don T Mix Two liquids that do not really mix well together, such as oil and water, are described as immiscible. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. Toluene is a common solvent in paint thinners and. What Three Liquids Don T Mix.
From www.elephango.com
Some Liquids Just Don't Mix Educational Resources K12 Learning What Three Liquids Don T Mix Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil and water don't mix. The components are said. What Three Liquids Don T Mix.
From www.slideshare.net
What can we observe about liquids What Three Liquids Don T Mix When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil and water don't mix. Two liquids that appear to mix completely together are said to be miscible. Gasoline. What Three Liquids Don T Mix.
From www.forever-mom.com
Why water and oil don’t mix? ForeverMom What Three Liquids Don T Mix Toluene is a common solvent in paint thinners and other industrial chemicals, and these typically mix poorly with water as well. Two liquids that appear to mix completely together are said to be miscible. When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is. What Three Liquids Don T Mix.
From www.reddit.com
The way these liquids don’t mix r/mildlyinteresting What Three Liquids Don T Mix Two liquids that do not really mix well together, such as oil and water, are described as immiscible. When two liquids combine to form a solution, they are called miscible. if they can't be combined, they are called immiscible. one example of this is oil (made from hydrogen and carbon) and water, which is the basis of the proverb, oil. What Three Liquids Don T Mix.
From www.youtube.com
Lying Liquids Liquids That Don't Mix SHORT YouTube What Three Liquids Don T Mix Liquids tend to be immiscible when the force of attraction between the molecules of the same liquid is greater than the force of attraction between the two different liquids. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. They form 3 layers with oil at the top, water in the middle and. What Three Liquids Don T Mix.
From www.youtube.com
Immiscible liquids Water and oil doesn't mix with each other Science What Three Liquids Don T Mix Two liquids that appear to mix completely together are said to be miscible. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. From least dense to most dense, the liquid layers are oil, water, dish. What Three Liquids Don T Mix.
From www.youtube.com
Oil and Water Don't Mix...Or Do They? YouTube What Three Liquids Don T Mix These three liquids do not mix easily, so when you shake. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. Two liquids that do not really mix well together, such as oil and water, are described. What Three Liquids Don T Mix.
From www.science-sparks.com
Why don't oil and water mix? Science Questions for Kids What Three Liquids Don T Mix They form 3 layers with oil at the top, water in the middle and vinegar at the bottom. These three liquids do not mix easily, so when you shake. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. The components are said to be immiscible. in contrast, fluids that do mix together. What Three Liquids Don T Mix.
From www.thoughtco.com
Why Oil and Water Don't Mix What Three Liquids Don T Mix From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. These three liquids do not mix easily, so when you shake. The components are said to be immiscible. in contrast, fluids that do mix together are called. What Three Liquids Don T Mix.
From tinyhousegarage.com
Why Don T Oil And Water Mix Chemistry? What Three Liquids Don T Mix These three liquids do not mix easily, so when you shake. Immiscibility is the property where two substances are not capable of combining to form a homogeneous mixture. Common examples include hexane (c6h14), toluene (c7h8) and cyclohexane (c6h12). From least dense to most dense, the liquid layers are oil, water, dish soap, and honey. Toluene is a common solvent in. What Three Liquids Don T Mix.
From www.shutterstock.com
Water Oil Mix Infographic Diagram Showing Stock Illustration 682213063 What Three Liquids Don T Mix Two liquids that appear to mix completely together are said to be miscible. These three liquids do not mix easily, so when you shake. Gasoline is a mixture of hydrocarbon solvents like hexane, which is why gasoline and water don't mix. The components are said to be immiscible. in contrast, fluids that do mix together are called miscible. When two. What Three Liquids Don T Mix.