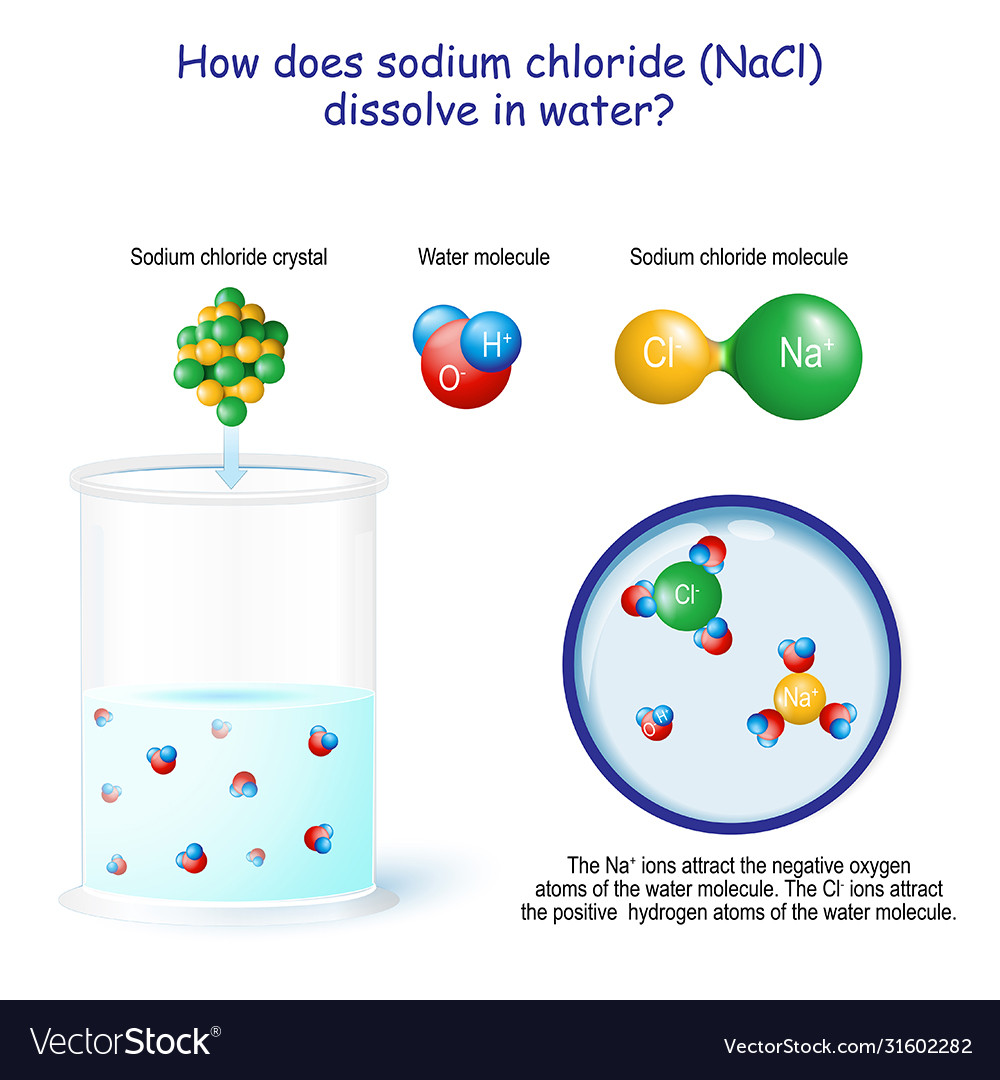
Why Do Crystals Dissolve In Water . We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The positively charged sodium ions in the crystal. Explain that in this activity, students will investigate how water dissolves salt crystals. Water is attracted to the sodium chloride crystal because water is polar; Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. They will also explore how the water from the salt solution evaporates, and how the. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. When a solute dissolves in water, the. It has both a positive and a negative end. The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by.
from www.bharatagritech.com
Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. Explain that in this activity, students will investigate how water dissolves salt crystals. The positively charged sodium ions in the crystal. When a solute dissolves in water, the. Water is attracted to the sodium chloride crystal because water is polar; It has both a positive and a negative end. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. They will also explore how the water from the salt solution evaporates, and how the. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves.
Ions In Aqueous Solution Infographic Diagram Showing, 54 OFF
Why Do Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. When a solute dissolves in water, the. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. It has both a positive and a negative end. The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by. They will also explore how the water from the salt solution evaporates, and how the. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. Explain that in this activity, students will investigate how water dissolves salt crystals. The positively charged sodium ions in the crystal. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. Water is attracted to the sodium chloride crystal because water is polar; Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves.
From byjus.com
Why ionic solids dissolve in water Why Do Crystals Dissolve In Water When a solute dissolves in water, the. Water is attracted to the sodium chloride crystal because water is polar; They will also explore how the water from the salt solution evaporates, and how the. Explain that in this activity, students will investigate how water dissolves salt crystals. It has both a positive and a negative end. Ionic compounds dissolve in. Why Do Crystals Dissolve In Water.
From courses.lumenlearning.com
Water Introduction to Biology Why Do Crystals Dissolve In Water Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. When a solute dissolves in water, the. We will first examine the process that occurs. Why Do Crystals Dissolve In Water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in Why Do Crystals Dissolve In Water The positively charged sodium ions in the crystal. Water is attracted to the sodium chloride crystal because water is polar; Explain that in this activity, students will investigate how water dissolves salt crystals. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Do Crystals Dissolve In Water It has both a positive and a negative end. They will also explore how the water from the salt solution evaporates, and how the. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. Water is attracted to the sodium chloride crystal because water is. Why Do Crystals Dissolve In Water.
From scitechdaily.com
New Research Reveals Liquid Crystals that Dissolve in Water Why Do Crystals Dissolve In Water The positively charged sodium ions in the crystal. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2948816 Why Do Crystals Dissolve In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. Explain that in this activity, students will investigate how water dissolves salt crystals. When a solute dissolves in water, the. They will also explore how the water from. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Introductory Chemistry , 3 rd Edition Nivaldo Tro PowerPoint Why Do Crystals Dissolve In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The positively charged sodium ions in the crystal. Water is attracted to the sodium chloride crystal because water is polar; When a solute dissolves in water, the. Because. Why Do Crystals Dissolve In Water.
From www.animalia-life.club
Crystallisation Of Copper Sulphate Why Do Crystals Dissolve In Water Water is attracted to the sodium chloride crystal because water is polar; Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. Explain that in this activity, students will investigate how water dissolves salt crystals. Ionic compounds dissolve in water if the energy given off when the ions interact with. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Do Crystals Dissolve In Water Water is attracted to the sodium chloride crystal because water is polar; It has both a positive and a negative end. The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility,. Why Do Crystals Dissolve In Water.
From chem.libretexts.org
Solubility and Precipitation Chemistry LibreTexts Why Do Crystals Dissolve In Water Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by. It has both a positive and a negative end. Ionic compounds dissolve in water if the energy given. Why Do Crystals Dissolve In Water.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Why Do Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The positively charged sodium ions in the. Why Do Crystals Dissolve In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Why Do Crystals Dissolve In Water Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. When a solute dissolves in water, the. Explain that in this activity, students will investigate how water dissolves salt crystals. The end result, then, is that in place of sodium chloride crystals, we have individual. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Why Do Crystals Dissolve In Water Water is attracted to the sodium chloride crystal because water is polar; Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. It has both a positive and a negative end. The positively charged sodium ions in the crystal. They will also explore how the water from the salt solution. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Why Do Crystals Dissolve In Water Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The positively charged. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Why Do Crystals Dissolve In Water The positively charged sodium ions in the crystal. They will also explore how the water from the salt solution evaporates, and how the. When a solute dissolves in water, the. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. We will first examine the. Why Do Crystals Dissolve In Water.
From slideplayer.com
Chapter 23 Solutions Lesson ppt download Why Do Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. It has both a positive and a negative end. Ionic compounds dissolve in water if the energy given off when. Why Do Crystals Dissolve In Water.
From exohvuzjm.blob.core.windows.net
Does Jelly Crystals Dissolve In Water at Richard Seese blog Why Do Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The positively charged sodium ions in the crystal. Explain that in this activity, students will investigate how water dissolves salt crystals. When a solute dissolves in water, the. Some crystals have high solubility, meaning they can dissolve readily in. Why Do Crystals Dissolve In Water.
From slidetodoc.com
HOW AND WHY DO SUBSTANCES DISSOLVE IN WATER Why Do Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. The positively charged sodium ions. Why Do Crystals Dissolve In Water.
From exyjtpwfh.blob.core.windows.net
Why Do Sodium Chloride Crystals Dissolve In Water at Tracy White blog Why Do Crystals Dissolve In Water The positively charged sodium ions in the crystal. Explain that in this activity, students will investigate how water dissolves salt crystals. When a solute dissolves in water, the. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. Ionic compounds dissolve in water if the. Why Do Crystals Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Why Do Crystals Dissolve In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. It has both a positive and a negative end. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves. Why Do Crystals Dissolve In Water.
From exohvuzjm.blob.core.windows.net
Does Jelly Crystals Dissolve In Water at Richard Seese blog Why Do Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. They will also explore how the water from the salt solution evaporates, and how the. The positively charged sodium ions in the crystal. Water is attracted to the sodium chloride crystal because water is polar; It has both a. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Why Do Crystals Dissolve In Water It has both a positive and a negative end. They will also explore how the water from the salt solution evaporates, and how the. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. The positively charged sodium ions in the crystal. Ionic compounds dissolve. Why Do Crystals Dissolve In Water.
From www.alamy.com
Potassium permanganate crystals dissolving in water Stock Photo Alamy Why Do Crystals Dissolve In Water The positively charged sodium ions in the crystal. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. Water is attracted to the sodium chloride crystal because water is polar; Explain that in this activity, students will investigate how water dissolves salt crystals. Ionic compounds. Why Do Crystals Dissolve In Water.
From crystalverse.com
The Extended Guide to Growing Copper Sulfate Crystals Why Do Crystals Dissolve In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. They will also explore how the water from the salt solution evaporates, and how the. Because the attractions of the water molecules for the sodium and chloride ions. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198 Why Do Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water is attracted to the sodium chloride crystal because water is polar; Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. Ionic compounds dissolve in water if the. Why Do Crystals Dissolve In Water.
From fineartamerica.com
Dissolving Copper Sulphate Crystals Photograph by Andrew Lambert Why Do Crystals Dissolve In Water It has both a positive and a negative end. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. When a solute dissolves in water, the. Water is attracted to the sodium chloride crystal because water is polar; We will first examine the process that occurs when an ionic compound. Why Do Crystals Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Why Do Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the. Water is attracted to the sodium chloride crystal because water is polar; Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. Because. Why Do Crystals Dissolve In Water.
From exohpqwmj.blob.core.windows.net
Which Crystals Dissolve In Water at Vivian Lamotte blog Why Do Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. Ionic compounds. Why Do Crystals Dissolve In Water.
From exohpqwmj.blob.core.windows.net
Which Crystals Dissolve In Water at Vivian Lamotte blog Why Do Crystals Dissolve In Water It has both a positive and a negative end. Explain that in this activity, students will investigate how water dissolves salt crystals. The positively charged sodium ions in the crystal. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. Some crystals have high solubility,. Why Do Crystals Dissolve In Water.
From www.bharatagritech.com
Ions In Aqueous Solution Infographic Diagram Showing, 54 OFF Why Do Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the. The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by. When a solute dissolves in water, the. Ionic compounds dissolve in water if the energy given off when the ions interact with water. Why Do Crystals Dissolve In Water.
From exohpqwmj.blob.core.windows.net
Which Crystals Dissolve In Water at Vivian Lamotte blog Why Do Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Do Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. Some crystals have high solubility, meaning they can dissolve readily in water, while. Why Do Crystals Dissolve In Water.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free Why Do Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the. Explain that in this activity, students will investigate how water dissolves salt crystals. Water is attracted to the sodium chloride crystal because water is polar; When a solute dissolves in water, the. We will first examine the process that occurs when an ionic compound such. Why Do Crystals Dissolve In Water.
From arianewsmcdowell.blogspot.com
Describe the Dissolving Process at the Molecular Level Why Do Crystals Dissolve In Water The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by. Some crystals have high solubility, meaning they can dissolve readily in water, while others have low solubility, requiring more time. Water is attracted to the sodium chloride crystal because water is polar; It has both a positive and. Why Do Crystals Dissolve In Water.
From damiannewsdixon.blogspot.com
Does Iodine Crystals Dissolve in Water Why Do Crystals Dissolve In Water The positively charged sodium ions in the crystal. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by. Some crystals have high solubility, meaning they can dissolve. Why Do Crystals Dissolve In Water.