Dilution Factor Weight/Volume . If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. Df = v f v i = 10.0ml 0.1ml = 100. Dilution factor is the factor by which the stock solution is diluted. Dilution factor = v f / v i in this formula, v f represents the final volume of the. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. It is often given as a ratio but can. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. The dilution factor can be calculated using the following formula:
from studylib.net
Dilution factor = v f / v i in this formula, v f represents the final volume of the. It is often given as a ratio but can. Dilution factor is the factor by which the stock solution is diluted. The dilution factor can be calculated using the following formula: Df = v f v i = 10.0ml 0.1ml = 100. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume.
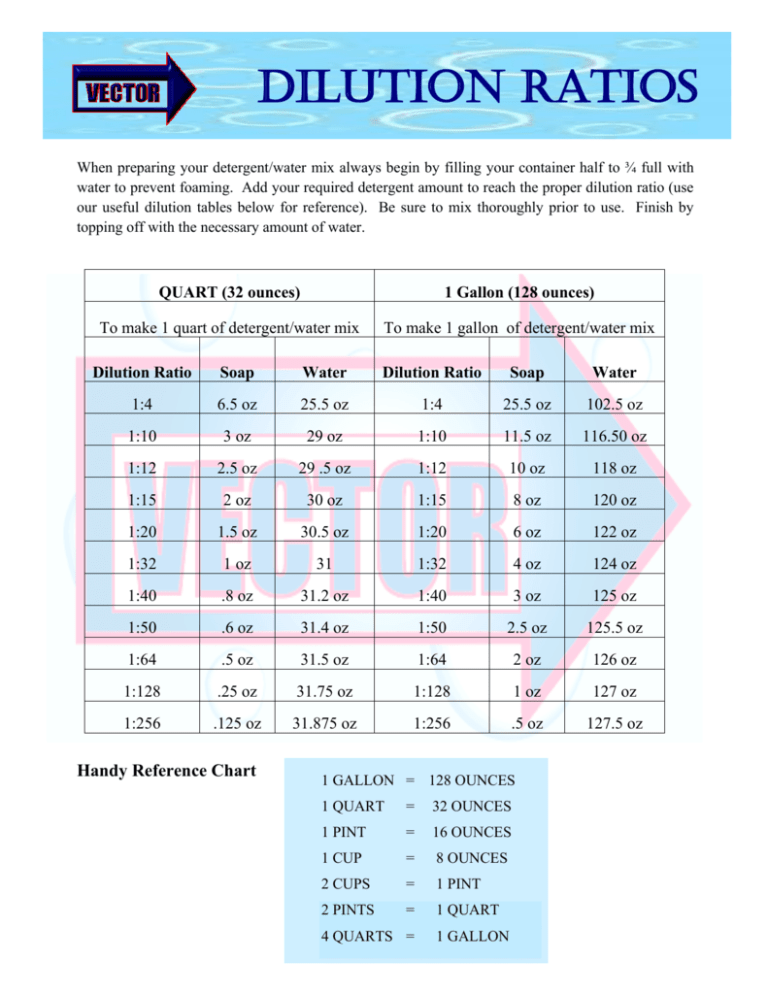
Dilution Ratios Table
Dilution Factor Weight/Volume The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. Dilution factor is the factor by which the stock solution is diluted. Dilution factor = v f / v i in this formula, v f represents the final volume of the. The dilution factor can be calculated using the following formula: It is often given as a ratio but can. Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free download ID1014345 Dilution Factor Weight/Volume If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. Dilution factor = v f / v i in this formula, v f represents the final volume of the. It is often given as a ratio but can. The dilution factor is calculated by dividing the initial volume of. Dilution Factor Weight/Volume.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID949751 Dilution Factor Weight/Volume Dilution factor = v f / v i in this formula, v f represents the final volume of the. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. It is often given as a ratio but can. The dilution factor is calculated. Dilution Factor Weight/Volume.
From www.researchgate.net
BCA assay dilution factor to divide or multiply? ResearchGate Dilution Factor Weight/Volume If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. Dilution factor is the factor by which the stock solution is diluted. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. The dilution factor (or dilution ratio). Dilution Factor Weight/Volume.
From www.bharatagritech.com
Calculating Dilution Factor, 50 OFF Dilution Factor Weight/Volume Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor is the factor by which the stock solution is diluted.. Dilution Factor Weight/Volume.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Volume Example 3 YouTube Dilution Factor Weight/Volume If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. Dilution factor is the factor by which the stock solution is diluted. Dilution factor = v f /. Dilution Factor Weight/Volume.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID1709844 Dilution Factor Weight/Volume Dilution factor is the factor by which the stock solution is diluted. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. The dilution factor can be calculated using the following formula: Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution factor or. Dilution Factor Weight/Volume.
From materialmagicpayne.z21.web.core.windows.net
How To Calculate Dilution Concentrations Dilution Factor Weight/Volume The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is calculated. Dilution Factor Weight/Volume.
From www.freepik.com
Premium Vector Dilution factor formula science vector illustration infographic Dilution Factor Weight/Volume The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Dilution factor is the factor by which the stock solution is diluted. It is often given as a ratio but can. The dilution factor is calculated by dividing the initial volume of the. Dilution Factor Weight/Volume.
From studylib.net
Dilution Ratios Table Dilution Factor Weight/Volume If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor. Dilution Factor Weight/Volume.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free download ID259008 Dilution Factor Weight/Volume Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. V f = aliquot volume + diluent volume =. Dilution Factor Weight/Volume.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Example 2 YouTube Dilution Factor Weight/Volume Dilution factor = v f / v i in this formula, v f represents the final volume of the. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. Dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume. Dilution Factor Weight/Volume.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Dilution Factor Weight/Volume Df = v f v i = 10.0ml 0.1ml = 100. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. The dilution factor. Dilution Factor Weight/Volume.
From www.omnicalculator.com
Dilution Factor Calculator Dilution Factor Weight/Volume If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. Dilution factor is the factor by which the stock solution is diluted. Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution factor or fold dilution calculations for solutions with mass per. Dilution Factor Weight/Volume.
From www.numerade.com
SOLVED Alternative way to solve dilution problems is to calculate the dilution factor (DF Dilution Factor Weight/Volume If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. It is often given as a ratio but can. Dilution factor = v f / v i in this formula, v f represents the final volume of the. Df = v f v i = 10.0ml 0.1ml = 100.. Dilution Factor Weight/Volume.
From www.researchgate.net
Uniform dilution with increasing dilution factor effect on gasliquid... Download Scientific Dilution Factor Weight/Volume Df = v f v i = 10.0ml 0.1ml = 100. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. The dilution factor can be calculated using the following formula: Dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume. Dilution Factor Weight/Volume.
From www.slideshare.net
Molarity and dilution Dilution Factor Weight/Volume The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution calculations for solutions with mass per volume or. Dilution Factor Weight/Volume.
From www.researchgate.net
The sample, dilution factor, and BOD values and the calculated... Download Scientific Diagram Dilution Factor Weight/Volume Dilution factor = v f / v i in this formula, v f represents the final volume of the. It is often given as a ratio but can. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. The dilution factor can be. Dilution Factor Weight/Volume.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution factor in Serial Dilution Factor Weight/Volume If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. The dilution factor can be calculated using the following formula: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. If you wish to perform dilution calculations for solutions with mass per volume. Dilution Factor Weight/Volume.
From support.axionbio.com
Understanding of Cell Sample Dilution Factor Dilution Factor Weight/Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. Dilution factor is the factor by which the stock solution is diluted.. Dilution Factor Weight/Volume.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Factor Weight/Volume The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. V f = aliquot volume + diluent volume =. Dilution Factor Weight/Volume.
From www.coursehero.com
[Solved] Exercise 3 Concentration, Solution, and Dilution Data Table 8.... Course Hero Dilution Factor Weight/Volume Dilution factor = v f / v i in this formula, v f represents the final volume of the. It is often given as a ratio but can. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. If you wish to perform dilution calculations for solutions with mass per volume or weight per. Dilution Factor Weight/Volume.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Factor Weight/Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor can be calculated using the following formula: If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. Dilution factor = v f / v i in this formula, v f. Dilution Factor Weight/Volume.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Factor Weight/Volume The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. It is often given as a ratio but can. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. The dilution factor can be. Dilution Factor Weight/Volume.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Factor Weight/Volume The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Df = v f v i = 10.0ml 0.1ml = 100. It is often given as a ratio but can. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml. Dilution Factor Weight/Volume.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free download ID259008 Dilution Factor Weight/Volume The dilution factor can be calculated using the following formula: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Df = v f v i = 10.0ml 0.1ml = 100. It is often given as a ratio but can. The dilution factor (or dilution ratio) is the notation used to express how much. Dilution Factor Weight/Volume.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Factor Weight/Volume Df = v f v i = 10.0ml 0.1ml = 100. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Dilution factor = v f / v i in this formula, v f represents the final volume of the. It is often. Dilution Factor Weight/Volume.
From www.youtube.com
Serial dilutions made simple YouTube Dilution Factor Weight/Volume The dilution factor can be calculated using the following formula: Df = v f v i = 10.0ml 0.1ml = 100. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. The dilution factor (or dilution ratio) is the. Dilution Factor Weight/Volume.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free download ID2225449 Dilution Factor Weight/Volume Dilution factor = v f / v i in this formula, v f represents the final volume of the. The dilution factor can be calculated using the following formula: If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. The dilution factor (or dilution ratio) is the notation used. Dilution Factor Weight/Volume.
From www.hemocytometer.org
Dilution factor calculator • Hemocytometer Dilution Factor Weight/Volume Dilution factor is the factor by which the stock solution is diluted. If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. The dilution factor can be calculated using the following formula: Dilution factor = v f / v i in this formula, v f represents the final volume. Dilution Factor Weight/Volume.
From www.pdfprof.com
how to calculate dilution factor Dilution Factor Weight/Volume It is often given as a ratio but can. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. V f =. Dilution Factor Weight/Volume.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Factor Weight/Volume Dilution factor is the factor by which the stock solution is diluted. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. Df = v f v i = 10.0ml 0.1ml = 100. It is often given as a ratio but can. Dilution factor = v f / v i in. Dilution Factor Weight/Volume.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Factor Weight/Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. Dilution factor = v f / v i in this formula, v f represents the final volume of the. If you wish to perform dilution. Dilution Factor Weight/Volume.
From www.slideshare.net
Molarity and dilution Dilution Factor Weight/Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Df = v f v i = 10.0ml 0.1ml = 100. Dilution factor = v f / v i in this formula, v f represents the final volume of the. If you wish to perform dilution calculations for solutions with mass per volume or. Dilution Factor Weight/Volume.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and chemistry YouTube Dilution Factor Weight/Volume Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume. Dilution factor is the factor by which the stock solution is diluted. The dilution factor (or dilution ratio) is the notation used to express how much of the. Dilution Factor Weight/Volume.
From www.transtutors.com
(Get Answer) Table 1 Serial Dilutions By A Factor Of 10 Volume (ML) Volume... Transtutors Dilution Factor Weight/Volume The dilution factor can be calculated using the following formula: If you wish to perform dilution calculations for solutions with mass per volume or weight per volume concentration units, use our. The dilution factor is calculated by dividing the initial volume of the solution by the final volume after dilution. Dilution factor is the factor by which the stock solution. Dilution Factor Weight/Volume.