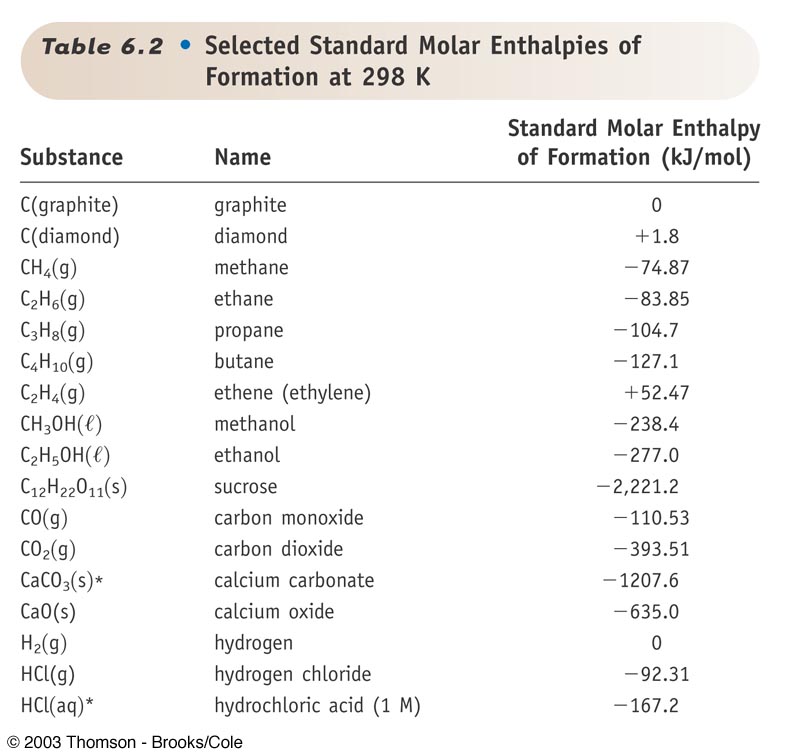
Standard Enthalpy Of Formation Values . A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Mallard, eds, nist chemistry webbook, nist standard reference database.
from lessonluft.z19.web.core.windows.net
A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Mallard, eds, nist chemistry webbook, nist standard reference database. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place).
Heat Of Formation Chart
Standard Enthalpy Of Formation Values Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Values Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Values.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Values A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. All standard state, 25 °c and 1 bar (written to 1 decimal place). Enthalpy of. Standard Enthalpy Of Formation Values.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Values Mallard, eds, nist chemistry webbook, nist standard reference database. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard. Standard Enthalpy Of Formation Values.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Enthalpy Of Formation Values A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for. Standard Enthalpy Of Formation Values.
From www.chegg.com
Solved ALL part of the same question ABased on the table of Standard Enthalpy Of Formation Values A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation Values.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Values A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. The standard enthalpy of formation, also known as the heat. Standard Enthalpy Of Formation Values.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation Values 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A standard enthalpy of formation $δh°_f$ is an. Standard Enthalpy Of Formation Values.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Enthalpy Of Formation Values A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and. Standard Enthalpy Of Formation Values.
From www.chegg.com
Using enthalpies of formation (Appendix C below, can Standard Enthalpy Of Formation Values The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Mallard, eds, nist chemistry webbook, nist standard reference database. Enthalpy. Standard Enthalpy Of Formation Values.
From radenbayu33as.blogspot.com
Enthalpy Of Formation Of C3H8 Enthalpy Changes during Formation of Steam Enthalpies of Standard Enthalpy Of Formation Values Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Enthalpy Of Formation Values.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Values Mallard, eds, nist chemistry webbook, nist standard reference database. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from. Standard Enthalpy Of Formation Values.
From brandonkss.github.io
Enthalpy Of Formation Chart Standard Enthalpy Of Formation Values 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation Values.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Enthalpy Of Formation Values Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of. Standard Enthalpy Of Formation Values.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Values Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Standard Enthalpy Of Formation Values.
From www.chegg.com
Using enthalpies of formation (Appendix C below, can Standard Enthalpy Of Formation Values A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows. Standard Enthalpy Of Formation Values.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Thermochemistry Unit Standard Enthalpy Of Formation Values The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation. Standard Enthalpy Of Formation Values.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation* for Various Compounds Standard Enthalpy Of Formation Values A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds, nist chemistry webbook, nist standard reference database. All standard. Standard Enthalpy Of Formation Values.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Enthalpy Of Formation Values All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation Values.
From www.researchgate.net
Standard Gibbs free energy of formation, standard enthalpy of formation... Download Table Standard Enthalpy Of Formation Values 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an. Standard Enthalpy Of Formation Values.
From www.researchgate.net
Enthalpy of formation, reaction, and of metal oxides and... Download Scientific Standard Enthalpy Of Formation Values The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. All standard state, 25 °c. Standard Enthalpy Of Formation Values.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Appendix I to calculate the Standard Enthalpy Of Formation Values Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. The standard enthalpy of formation, also known as the heat. Standard Enthalpy Of Formation Values.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Values 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy. Standard Enthalpy Of Formation Values.
From www.chegg.com
Solved Using either the two equations below, or Heats of Standard Enthalpy Of Formation Values A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. All. Standard Enthalpy Of Formation Values.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation Values Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. Mallard, eds, nist chemistry webbook, nist standard reference database. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Standard Enthalpy Of Formation Values.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Enthalpy Of Formation Values Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpy Of Formation Values.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Values A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation Values.
From pt.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Values All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound. Standard Enthalpy Of Formation Values.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID6642303 Standard Enthalpy Of Formation Values 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an. Standard Enthalpy Of Formation Values.
From www.numerade.com
SOLVED Calculate the standard enthalpy change for the reaction at 25 ∘25 ∘C. Standard enthalpy Standard Enthalpy Of Formation Values Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Mallard, eds,. Standard Enthalpy Of Formation Values.
From schoolworkhelper.net
StandardEnthalpiesFormation Standard Enthalpy Of Formation Values 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Values.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Values The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar. Standard Enthalpy Of Formation Values.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Values All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such. Standard Enthalpy Of Formation Values.
From www.researchgate.net
Heat of formation and enthalpy data for slag compounds Enthalpy of... Download Table Standard Enthalpy Of Formation Values Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. All standard state, 25 °c and. Standard Enthalpy Of Formation Values.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Values Mallard, eds, nist chemistry webbook, nist standard reference database. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. The. Standard Enthalpy Of Formation Values.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work... Download Table Standard Enthalpy Of Formation Values The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide. All standard. Standard Enthalpy Of Formation Values.