Emission Spectra Theory . learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. See examples of emission spectra of hydrogen,. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how each element has a unique spectrum of light frequencies that can be used to identify them.
from stock.adobe.com
learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how each element has a unique spectrum of light frequencies that can be used to identify them. See examples of emission spectra of hydrogen,. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom.
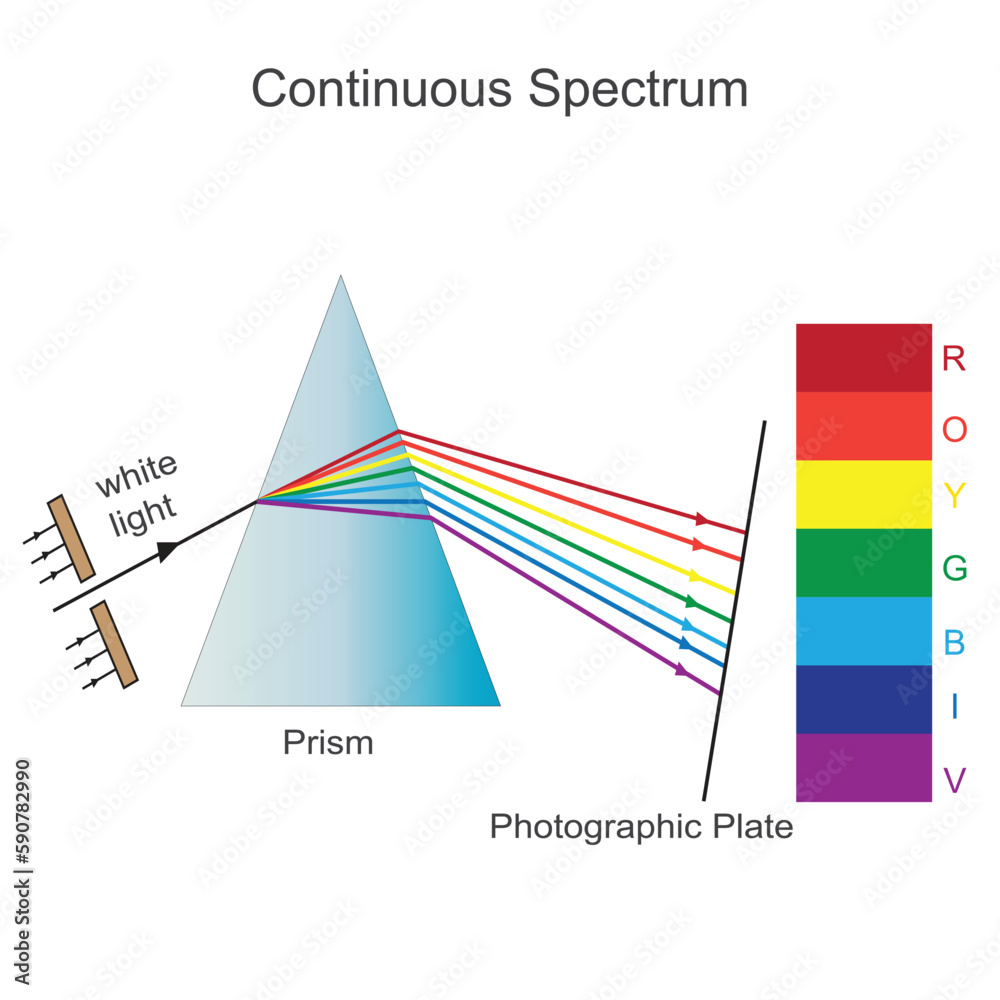
Continuous spectrum, an emission spectrum that consists of continuum of
Emission Spectra Theory the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how each element has a unique spectrum of light frequencies that can be used to identify them. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. See examples of emission spectra of hydrogen,.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Emission Spectra Theory learn how each element has a unique spectrum of light frequencies that can be used to identify them. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. See examples of emission spectra. Emission Spectra Theory.
From www.researchgate.net
Emission spectra of the sample IPM80 at T = 78 K. 1 CW excitation Emission Spectra Theory learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how each element has a unique spectrum of light frequencies that can be used to identify them. learn how. Emission Spectra Theory.
From www.researchgate.net
Emission spectra of Ca2YNbO6Mn annealed in different atmospheric Emission Spectra Theory learn how each element has a unique spectrum of light frequencies that can be used to identify them. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them.. Emission Spectra Theory.
From socratic.org
How did Bohr theory explain the emission spectrum of hydrogen? Socratic Emission Spectra Theory learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. the atomic. Emission Spectra Theory.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Emission Spectra Theory See examples of emission spectra of hydrogen,. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how each element has a unique spectrum of light frequencies that. Emission Spectra Theory.
From www.researchgate.net
Emission spectra of ADR1 dye with glucose. 1) Emission spectra of ADR1 Emission Spectra Theory the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through. Emission Spectra Theory.
From stock.adobe.com
Continuous spectrum, an emission spectrum that consists of continuum of Emission Spectra Theory learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how atoms emit light of. Emission Spectra Theory.
From www.researchgate.net
Figure S9. Emission spectra of complex 4 in CH 3 CN at different Emission Spectra Theory learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how each element has a unique spectrum of light frequencies that can be used to identify them. a characteristic pattern. Emission Spectra Theory.
From chem.libretexts.org
4.4 Bohr's Theory of the Hydrogen Emission Spectrum Chemistry LibreTexts Emission Spectra Theory learn how each element has a unique spectrum of light frequencies that can be used to identify them. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn how hydrogen. Emission Spectra Theory.
From pressbooks.bccampus.ca
2.3 Bohr’s Theory of the Hydrogen Atom Atomic Spectral Lines Emission Spectra Theory learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. See examples of emission spectra of hydrogen,. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how the emission spectra of hydrogen and other elements reveal. Emission Spectra Theory.
From chem.libretexts.org
11.4 Bohr's Theory of the Hydrogen Emission Spectrum Chemistry Emission Spectra Theory learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. See examples. Emission Spectra Theory.
From www.researchgate.net
(A,B) Emission spectra of 1 × 10⁻⁵ M of compounds (a) 3, (b) 5 exciting Emission Spectra Theory a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. learn about the. Emission Spectra Theory.
From www.youtube.com
2.2 Hydrogen emission spectrum (SL) YouTube Emission Spectra Theory See examples of emission spectra of hydrogen,. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn about the unique patterns of light emitted or absorbed by atoms when their electrons. Emission Spectra Theory.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Theory learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. See examples of emission. Emission Spectra Theory.
From www.pinterest.com
Emission Spectra and the Bohr Model Bohr model, Emissions, Model Emission Spectra Theory learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. a. Emission Spectra Theory.
From www.researchgate.net
(a) Emission spectra of 11, 12, and 13 in 5 wt PMMA films at 298 K Emission Spectra Theory the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. learn how each element has a unique spectrum of light frequencies that can be used to identify them. . Emission Spectra Theory.
From www.researchgate.net
Emission spectra of TC with various concentrations of Zn 2+ The Emission Spectra Theory See examples of emission spectra of hydrogen,. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. the atomic spectrum of hydrogen and the spectra of many other species. Emission Spectra Theory.
From www.researchgate.net
Emission spectra and DFT energies of the doublet (D1D3) states of the Emission Spectra Theory learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. a characteristic. Emission Spectra Theory.
From www.slideserve.com
PPT Atomic spectra are a result of energy level diagrams quantum Emission Spectra Theory learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how each element has a unique spectrum of light frequencies that can be used to identify them. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn how. Emission Spectra Theory.
From chemcollective.org
CHEM1315 Lab 8 Atomic Spectrum Emission Spectra Theory the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy. Emission Spectra Theory.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Theory the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how each element has a unique spectrum of light frequencies that can be used to identify them.. Emission Spectra Theory.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Theory See examples of emission spectra of hydrogen,. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. the atomic spectrum of hydrogen and the spectra of many other. Emission Spectra Theory.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation ID6909895 Emission Spectra Theory learn how each element has a unique spectrum of light frequencies that can be used to identify them. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels.. Emission Spectra Theory.
From www.researchgate.net
(a) Normalized emission, (b) actual emission, and (c) PLE spectra of Emission Spectra Theory a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. the atomic spectrum of hydrogen and. Emission Spectra Theory.
From dxoxckuai.blob.core.windows.net
Emission Spectra Of Hydrogen Atom at James Kraemer blog Emission Spectra Theory learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. See examples of emission spectra of hydrogen,. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence. Emission Spectra Theory.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Theory learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. learn how each element has a unique spectrum of light frequencies that can be used to identify them. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. a characteristic pattern. Emission Spectra Theory.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectra Theory a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how hydrogen. Emission Spectra Theory.
From www.researchgate.net
Steady state absorption and emission spectra of PXFCN in solvents of Emission Spectra Theory learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. See examples of emission spectra of hydrogen,. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited state.. Emission Spectra Theory.
From www.slideserve.com
PPT “Light and Reflection” PowerPoint Presentation ID1908822 Emission Spectra Theory See examples of emission spectra of hydrogen,. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how atoms emit light of specific wavelengths when they return. Emission Spectra Theory.
From www.researchgate.net
(a) Excitation spectrum of Eu 3+ line emission at 610 nm and excitation Emission Spectra Theory learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn how atoms emit light of specific wavelengths when they return to the ground state from an excited. Emission Spectra Theory.
From www.researchgate.net
Typical PL excitation spectra and emission spectra for... Download Emission Spectra Theory learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how each element has a unique spectrum of light frequencies that can be used to identify them.. Emission Spectra Theory.
From www.researchgate.net
a emission spectra; b normalized emission spectra; c UV spectrum (left Emission Spectra Theory learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how each element has a unique spectrum of light frequencies that can be used to identify them. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of.. Emission Spectra Theory.
From www.slideserve.com
PPT EMISSION AND ABSORPTION SPECTRA PowerPoint Presentation, free Emission Spectra Theory a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. the atomic spectrum of hydrogen and the spectra of many other species provided major evidence for the quantization of. learn how atoms emit light of. Emission Spectra Theory.
From goc-oivf2.blogspot.com
40 emission spectra and energy levels worksheet Worksheet Information Emission Spectra Theory a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. learn how the emission spectra of hydrogen and other elements reveal the quantized energy levels of. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. learn how each element has. Emission Spectra Theory.
From www.researchgate.net
normalized emission spectra of complexes 13 in the solid state at (a Emission Spectra Theory learn how hydrogen atoms emit light of specific wavelengths and colors when an electric current is passed through them. learn about the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. a characteristic pattern of spectral lines, either absorption or emission, produced by the hydrogen atom. the atomic. Emission Spectra Theory.