Examples Of Emission Spectroscopy . This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. However, when separated using a prism or diffraction grating, the. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Also, emission spectra are used to identify. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For example, by studying emission spectra of the stars, we can determine their chemical composition.
from microbiologynotes.org
Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Also, emission spectra are used to identify. For example, by studying emission spectra of the stars, we can determine their chemical composition. However, when separated using a prism or diffraction grating, the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence.
uv vis spectroscopy Microbiology Notes
Examples Of Emission Spectroscopy For example, by studying emission spectra of the stars, we can determine their chemical composition. For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used to identify. However, when separated using a prism or diffraction grating, the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Examples Of Emission Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. However, when separated using a prism or diffraction grating, the. For example, by studying emission spectra of the stars, we can determine their chemical composition. The release of a photon following thermal excitation is called emission and that following the. Examples Of Emission Spectroscopy.
From medium.com
Light and Dark Understanding Spectrometry by Amelia Settembre Medium Examples Of Emission Spectroscopy The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. However, when separated using a prism or diffraction grating, the. Also, emission spectra are used to identify. For example, by studying emission spectra of the stars, we can determine their chemical composition. The emission spectrum (or line spectrum). Examples Of Emission Spectroscopy.
From www.savemyexams.com
Flame Emission Spectroscopy AQA GCSE Chemistry Revision Notes 2018 Examples Of Emission Spectroscopy When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For example, by studying emission spectra of the stars, we can determine their chemical composition. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. Classical emission. Examples Of Emission Spectroscopy.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Examples Of Emission Spectroscopy For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used to identify. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. When hydrogen gas is placed into a tube and electric current passed through it, the color. Examples Of Emission Spectroscopy.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Examples Of Emission Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Examples Of Emission Spectroscopy.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Examples Of Emission Spectroscopy For example, by studying emission spectra of the stars, we can determine their chemical composition. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or. Examples Of Emission Spectroscopy.
From www.pinterest.com
Absorption Spectroscopy Absorption spectroscopy translations • Free Examples Of Emission Spectroscopy When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For example, by studying emission spectra of the stars, we can determine their chemical composition. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. This spectrum of radiation. Examples Of Emission Spectroscopy.
From www.slideshare.net
Emission spectroscopy Examples Of Emission Spectroscopy For example, by studying emission spectra of the stars, we can determine their chemical composition. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light. Examples Of Emission Spectroscopy.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Examples Of Emission Spectroscopy This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Examples Of Emission Spectroscopy.
From crunchchemistry.co.uk
Atomic absorption and emission spectroscopy Crunch Chemistry Examples Of Emission Spectroscopy The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. This spectrum of radiation emitted by electrons in the excited atoms or molecules is. Examples Of Emission Spectroscopy.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Examples Of Emission Spectroscopy The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Also, emission spectra are used to identify. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. When hydrogen gas is placed. Examples Of Emission Spectroscopy.
From www.slideserve.com
PPT Atomic Spectroscopy Laboratory PowerPoint Presentation, free Examples Of Emission Spectroscopy Also, emission spectra are used to identify. For example, by studying emission spectra of the stars, we can determine their chemical composition. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. However, when separated using a prism or diffraction grating, the. When hydrogen gas is placed into a tube and. Examples Of Emission Spectroscopy.
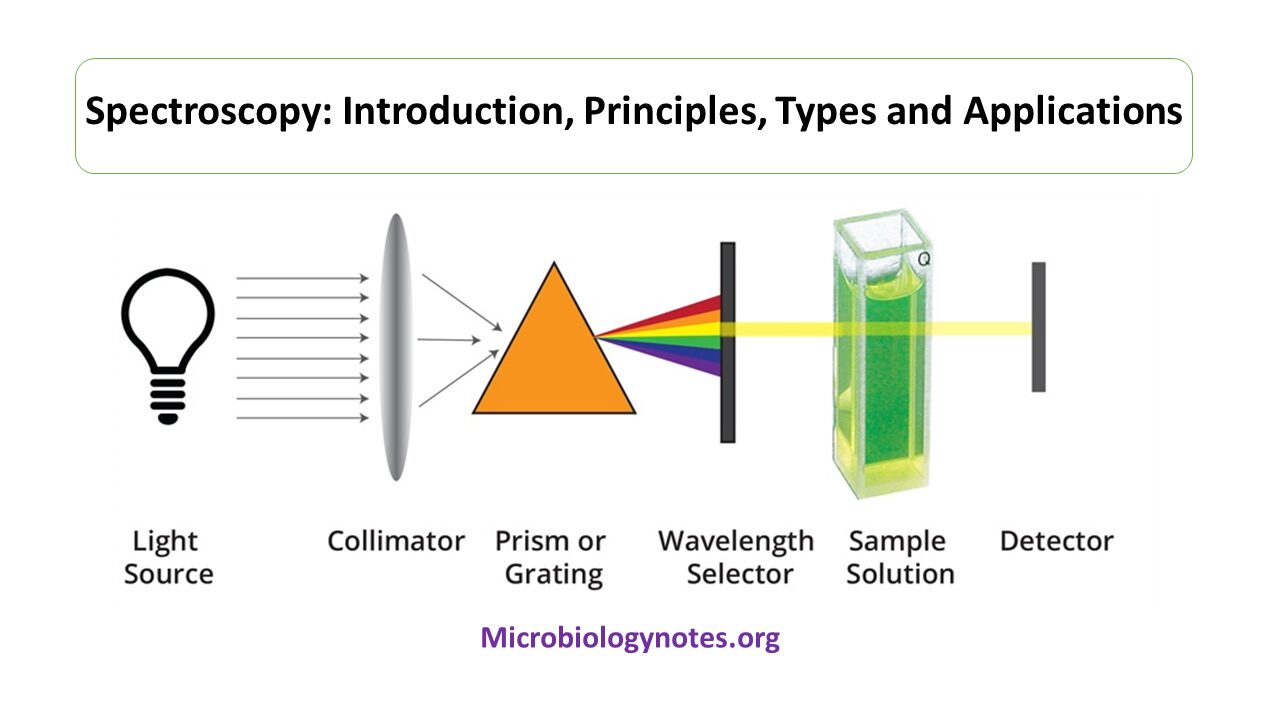
From microbiologynotes.org
Spectroscopy Introduction, Principles, Types and Applications Examples Of Emission Spectroscopy For example, by studying emission spectra of the stars, we can determine their chemical composition. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Also, emission. Examples Of Emission Spectroscopy.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Examples Of Emission Spectroscopy For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used to identify. However, when separated using a prism or diffraction grating, the. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. Classical emission spectroscopy is based on. Examples Of Emission Spectroscopy.
From www.slideserve.com
PPT Principle of Emission Spectroscopy I PowerPoint Presentation Examples Of Emission Spectroscopy When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Also, emission spectra are used to identify. However, when separated using a prism or diffraction grating, the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Examples Of Emission Spectroscopy.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Examples Of Emission Spectroscopy The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. Also, emission spectra are used to identify. Classical emission spectroscopy is based. Examples Of Emission Spectroscopy.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Examples Of Emission Spectroscopy For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used to identify. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted. Examples Of Emission Spectroscopy.
From www.astronoo.com
Spectroscopy — Astronoo Examples Of Emission Spectroscopy Also, emission spectra are used to identify. However, when separated using a prism or diffraction grating, the. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For example, by studying emission spectra of the stars, we can determine their chemical composition. Classical emission spectroscopy is based on excitation. Examples Of Emission Spectroscopy.
From www.slideserve.com
PPT Principle of Emission Spectroscopy I PowerPoint Presentation Examples Of Emission Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. However, when separated using a prism or diffraction grating, the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Also, emission spectra are used to identify. The emission spectrum (or. Examples Of Emission Spectroscopy.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Examples Of Emission Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. For example, by studying emission spectra of the stars, we can determine their chemical composition. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. However, when separated using a prism. Examples Of Emission Spectroscopy.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Examples Of Emission Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. Also, emission spectra are used to identify. However, when separated using a prism or diffraction grating, the. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. The. Examples Of Emission Spectroscopy.
From www.youtube.com
Atomic Emission Spectroscopy YouTube Examples Of Emission Spectroscopy The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. For example, by studying emission spectra of the stars, we can determine their chemical composition. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in. Examples Of Emission Spectroscopy.
From www.youtube.com
ATOMIC EMISSION SPECTROSCOPY Flame Photometry Instrumentation Examples Of Emission Spectroscopy When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Also, emission spectra are used to identify. For example, by studying emission spectra. Examples Of Emission Spectroscopy.
From www.researchgate.net
Optical emission spectroscopy emission spectra within the range of Examples Of Emission Spectroscopy This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Examples Of Emission Spectroscopy.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Examples Of Emission Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used to identify. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when. Examples Of Emission Spectroscopy.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Examples Of Emission Spectroscopy However, when separated using a prism or diffraction grating, the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. The release of a photon following thermal excitation is called emission. Examples Of Emission Spectroscopy.
From www.researchgate.net
Optical emission spectroscopy spectrum of dielectric barrier discharge Examples Of Emission Spectroscopy However, when separated using a prism or diffraction grating, the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The release of a photon. Examples Of Emission Spectroscopy.
From slideplayer.com
Molecular Spectroscopy ppt download Examples Of Emission Spectroscopy This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is. Examples Of Emission Spectroscopy.
From www.researchgate.net
Schematic representation of atomic emission spectroscopy (AES) with Examples Of Emission Spectroscopy Also, emission spectra are used to identify. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. The emission spectrum (or line spectrum) of a chemical element. Examples Of Emission Spectroscopy.
From microbiologynotes.org
uv vis spectroscopy Microbiology Notes Examples Of Emission Spectroscopy This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. However, when separated using a prism or diffraction grating, the. For example, by studying emission spectra of the stars, we. Examples Of Emission Spectroscopy.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Examples Of Emission Spectroscopy When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Examples Of Emission Spectroscopy.
From users.highland.edu
Atomic Spectra and Models of the Atom Examples Of Emission Spectroscopy However, when separated using a prism or diffraction grating, the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used to identify. When hydrogen gas is placed into a tube and. Examples Of Emission Spectroscopy.
From joihsprvv.blob.core.windows.net
Emission Spectra Bbc Bitesize at Jose Doty blog Examples Of Emission Spectroscopy Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The release of a photon following thermal excitation is called emission and that following. Examples Of Emission Spectroscopy.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Examples Of Emission Spectroscopy This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. However, when separated using a prism or diffraction grating, the. Also, emission spectra are used to identify. The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. Classical emission. Examples Of Emission Spectroscopy.
From www.researchgate.net
CO 2 optical emission spectroscopy (OES) (a) Schematic of the Examples Of Emission Spectroscopy The release of a photon following thermal excitation is called emission and that following the absorption of a photon is called photoluminescence. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by electron impact (in gas. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted. Examples Of Emission Spectroscopy.