Why Does Alcohol Dissolve In Water . Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. The small alcohols are completely soluble in water. Alcohols can be considered derivatives of water (h 2 o; In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Whatever proportions you mix them in, you will get a single solution. The alcohol molecules form hydrogen. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Solubility of alcohols in water.
from www.nagwa.com
However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Solubility of alcohols in water. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Mixing the two in any proportion generates a single solution. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The alcohol molecules form hydrogen. Whatever proportions you mix them in, you will get a single solution. The small alcohols are completely soluble in water. Alcohols can be considered derivatives of water (h 2 o;
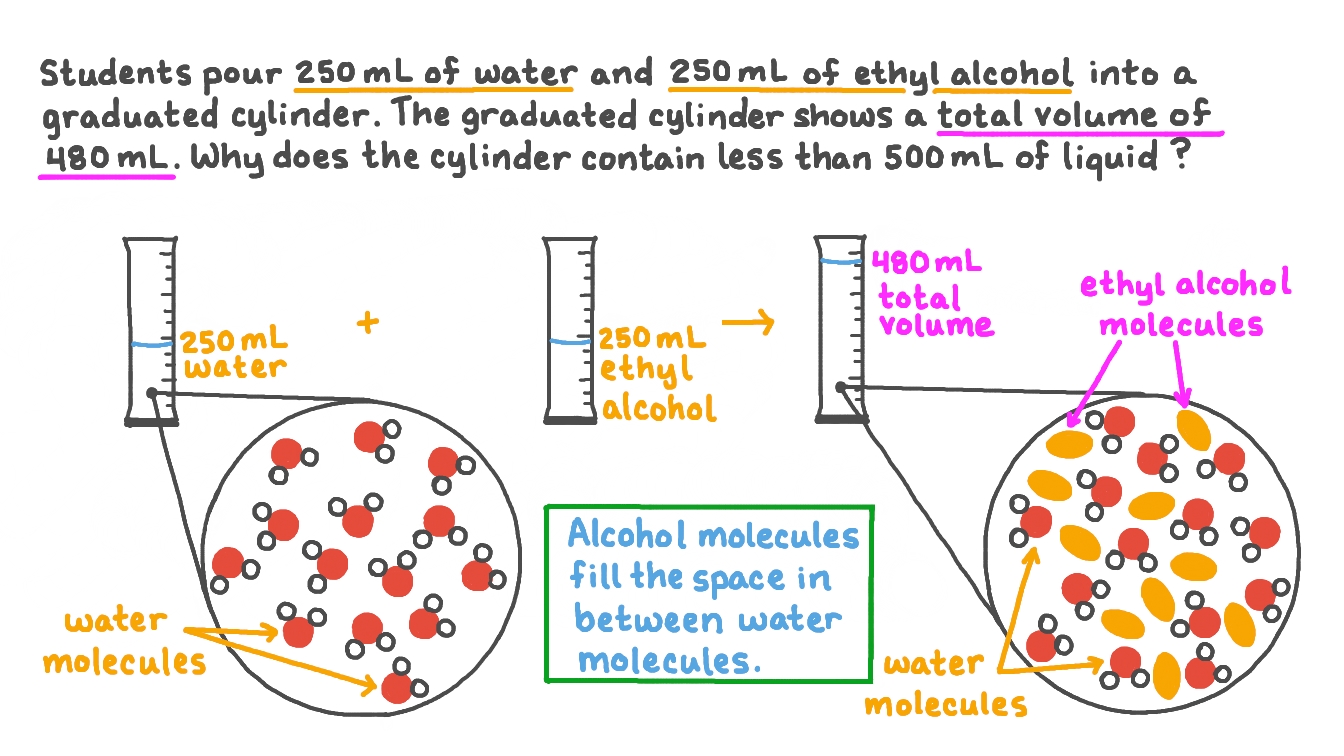
Question Video Explaining Why the Volume of an Alcohol and Water
Why Does Alcohol Dissolve In Water Small alcohols are completely soluble in water; Alcohols can be considered derivatives of water (h 2 o; Small alcohols are completely soluble in water; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Whatever proportions you mix them in, you will get a single solution. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. The alcohol molecules form hydrogen. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; Solubility of alcohols in water. Mixing the two in any proportion generates a single solution. The small alcohols are completely soluble in water.
From www.slideserve.com
PPT Solutions and their Behavior PowerPoint Presentation, free Why Does Alcohol Dissolve In Water Whatever proportions you mix them in, you will get a single solution. The alcohol molecules form hydrogen. Alcohols can be considered derivatives of water (h 2 o; However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Solubility of alcohols in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in. Why Does Alcohol Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Does Alcohol Dissolve In Water Small alcohols are completely soluble in water; However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Mixing the two in any proportion generates a single solution. Whatever proportions you mix them in, you will get a single solution. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in. Why Does Alcohol Dissolve In Water.
From www.slideserve.com
PPT Physical and Chemical Properties Of Alcohols! PowerPoint Why Does Alcohol Dissolve In Water However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Solubility of alcohols in water. Mixing the two in any proportion generates a single solution. The small alcohols are completely soluble in water. Whatever proportions you mix them in, you will get a single solution. Alcohols of four or fewer carbon atoms are soluble in water. Why Does Alcohol Dissolve In Water.
From www.nagwa.com
Question Video Explaining Why the Volume of an Alcohol and Water Why Does Alcohol Dissolve In Water The alcohol molecules form hydrogen. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. The small alcohols are completely soluble in water. Whatever proportions you mix them in, you will get a single. Why Does Alcohol Dissolve In Water.
From www.youtube.com
Solubility of Alcohol in Water Part 1 YouTube Why Does Alcohol Dissolve In Water Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The alcohol molecules form hydrogen. The small alcohols are completely soluble in water. Small alcohols are completely. Why Does Alcohol Dissolve In Water.
From slideplayer.com
melting & boiling points ppt download Why Does Alcohol Dissolve In Water In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen. Why Does Alcohol Dissolve In Water.
From www.youtube.com
Linear Molecules of PVA dissolve in water . Lecture no 14 . YouTube Why Does Alcohol Dissolve In Water The alcohol molecules form hydrogen. The small alcohols are completely soluble in water. Small alcohols are completely soluble in water; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Whatever proportions you mix them in, you will get a single solution. Alcohols can be considered derivatives of water. Why Does Alcohol Dissolve In Water.
From www.quanswer.com
Why alcohol dissolve in water? Quanswer Why Does Alcohol Dissolve In Water The alcohol molecules form hydrogen. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Solubility of alcohols in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The small alcohols are completely soluble in water.. Why Does Alcohol Dissolve In Water.
From www.chemistrystudent.com
Alcohols (ALevel) ChemistryStudent Why Does Alcohol Dissolve In Water Solubility of alcohols in water. Mixing the two in any proportion generates a single solution. The alcohol molecules form hydrogen. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Small alcohols are completely soluble in water; Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding. Why Does Alcohol Dissolve In Water.
From www.youtube.com
Physical Properties of Alcohol Hydrogen Bonding, Solubility and Why Does Alcohol Dissolve In Water Whatever proportions you mix them in, you will get a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The alcohol molecules form hydrogen. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Mixing. Why Does Alcohol Dissolve In Water.
From recoveryranger.com
How Do You Separate Alcohol And Water? Recovery Ranger Why Does Alcohol Dissolve In Water Small alcohols are completely soluble in water; Alcohols can be considered derivatives of water (h 2 o; Solubility of alcohols in water. The alcohol molecules form hydrogen. The small alcohols are completely soluble in water. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Mixing the two in. Why Does Alcohol Dissolve In Water.
From www.numerade.com
SOLVED ALCOHOLS, PHENOLS CARBOXYLIC ACIDS Solubility in Water (cross Why Does Alcohol Dissolve In Water Solubility of alcohols in water. Alcohols can be considered derivatives of water (h 2 o; However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The small alcohols are completely soluble in water. The alcohol. Why Does Alcohol Dissolve In Water.
From yesdirt.com
Does Alcohol Dissolve in Water? (ANSWERED) Yes Dirt Why Does Alcohol Dissolve In Water Alcohols can be considered derivatives of water (h 2 o; Small alcohols are completely soluble in water; In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Whatever proportions you mix them in, you will get a single solution. However, solubility decreases as the length of the hydrocarbon chain. Why Does Alcohol Dissolve In Water.
From www.youtube.com
Can Liquids Dissolve in Water? YouTube Why Does Alcohol Dissolve In Water Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable. Why Does Alcohol Dissolve In Water.
From www.acs.org
Lesson 5.3 Why Does Water Dissolve Salt? American Chemical Society Why Does Alcohol Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Solubility of alcohols in water. The small alcohols are completely soluble in water. Whatever proportions you mix them in, you will get a single solution. Small alcohols are completely soluble in water; Mixing the two in any proportion generates. Why Does Alcohol Dissolve In Water.
From loegaqddj.blob.core.windows.net
Alcohol Dissolve In Water Or Not at Monica blog Why Does Alcohol Dissolve In Water The small alcohols are completely soluble in water. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; Whatever proportions you mix them in, you will get a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes. Why Does Alcohol Dissolve In Water.
From klalyxthl.blob.core.windows.net
Does Denatured Alcohol Dissolve In Water at Josephine Jacobs blog Why Does Alcohol Dissolve In Water Whatever proportions you mix them in, you will get a single solution. Solubility of alcohols in water. Alcohols can be considered derivatives of water (h 2 o; The alcohol molecules form hydrogen. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. The small alcohols are completely soluble in water. In case of alcohols, just as. Why Does Alcohol Dissolve In Water.
From www.slideserve.com
PPT 2 Intermolecular forces PowerPoint Presentation, free download Why Does Alcohol Dissolve In Water However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Small alcohols are completely soluble in water; Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen. Why Does Alcohol Dissolve In Water.
From www.youtube.com
Is C2H5OH (Ethanol) Soluble or Insoluble in Water? YouTube Why Does Alcohol Dissolve In Water In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Whatever proportions you mix them in, you will get a single solution. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Solubility of alcohols in water. Alcohols of four or fewer carbon atoms are. Why Does Alcohol Dissolve In Water.
From www.youtube.com
Polyvinyl alcohol PVA dissolving in water timelapse YouTube Why Does Alcohol Dissolve In Water Whatever proportions you mix them in, you will get a single solution. Solubility of alcohols in water. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble.. Why Does Alcohol Dissolve In Water.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 2 activity for kids Why Does Alcohol Dissolve In Water The alcohol molecules form hydrogen. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. The small alcohols are completely soluble in water. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Alcohols can be considered derivatives of water (h 2 o; Solubility of. Why Does Alcohol Dissolve In Water.
From www.slideshare.net
Alcohol powerpoint Why Does Alcohol Dissolve In Water The small alcohols are completely soluble in water. Small alcohols are completely soluble in water; Whatever proportions you mix them in, you will get a single solution. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. The alcohol molecules form hydrogen. Solubility of alcohols in water. Mixing the two in any proportion generates a single. Why Does Alcohol Dissolve In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 Why Does Alcohol Dissolve In Water In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Whatever proportions you mix them in, you will get a single solution. Mixing the two in any proportion generates a single solution. The alcohol molecules form hydrogen. Alcohols can be considered derivatives of water (h 2 o; Explain why. Why Does Alcohol Dissolve In Water.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Why Does Alcohol Dissolve In Water Alcohols can be considered derivatives of water (h 2 o; The alcohol molecules form hydrogen. Solubility of alcohols in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; The small alcohols are completely soluble in water. However, solubility decreases as. Why Does Alcohol Dissolve In Water.
From loecasjgq.blob.core.windows.net
What Substance Can Water Dissolve at Leo Slack blog Why Does Alcohol Dissolve In Water Solubility of alcohols in water. Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Alcohols can be considered derivatives of water (h 2 o; In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. The small alcohols are completely soluble in. Why Does Alcohol Dissolve In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Why Does Alcohol Dissolve In Water The alcohol molecules form hydrogen. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Whatever proportions you mix them in, you will get a single solution.. Why Does Alcohol Dissolve In Water.
From loegaqddj.blob.core.windows.net
Alcohol Dissolve In Water Or Not at Monica blog Why Does Alcohol Dissolve In Water Solubility of alcohols in water. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule. Why Does Alcohol Dissolve In Water.
From www.slideshare.net
Introduction of alcohol Why Does Alcohol Dissolve In Water In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Mixing the two in any proportion generates a single solution. Alcohols can be considered derivatives of water (h 2 o; Solubility of alcohols in water.. Why Does Alcohol Dissolve In Water.
From www.youtube.com
4 2 4 alcohols solubility and solvent polarity YouTube Why Does Alcohol Dissolve In Water In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. The small alcohols are completely soluble in water. Alcohols can be considered derivatives of water (h 2 o; However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Alcohols of four or fewer carbon atoms. Why Does Alcohol Dissolve In Water.
From www.thesciencehive.co.uk
Alcohols* — the science sauce Why Does Alcohol Dissolve In Water Small alcohols are completely soluble in water; The alcohol molecules form hydrogen. Whatever proportions you mix them in, you will get a single solution. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Mixing. Why Does Alcohol Dissolve In Water.
From loegaqddj.blob.core.windows.net
Alcohol Dissolve In Water Or Not at Monica blog Why Does Alcohol Dissolve In Water The alcohol molecules form hydrogen. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; The small alcohols are completely soluble in water. Alcohols can be considered derivatives of water (h 2 o; Solubility of alcohols in water. In case of alcohols, just as it happens in case. Why Does Alcohol Dissolve In Water.
From scholarsark.com
How do you seperate alcohol from water Scholars Ark Why Does Alcohol Dissolve In Water The small alcohols are completely soluble in water. Alcohols can be considered derivatives of water (h 2 o; In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water. Why Does Alcohol Dissolve In Water.
From klalyxthl.blob.core.windows.net
Does Denatured Alcohol Dissolve In Water at Josephine Jacobs blog Why Does Alcohol Dissolve In Water Mixing the two in any proportion generates a single solution. The alcohol molecules form hydrogen. Whatever proportions you mix them in, you will get a single solution. Alcohols can be considered derivatives of water (h 2 o; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. However, solubility. Why Does Alcohol Dissolve In Water.
From saylordotorg.github.io
Physical Properties of Alcohols Why Does Alcohol Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The small alcohols are completely soluble in water. Small alcohols are completely soluble in water; However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. Alcohols of four or fewer carbon atoms are soluble in. Why Does Alcohol Dissolve In Water.
From slidetodoc.com
Chapter 13 Alcohols Phenols and Thiols 13 3 Why Does Alcohol Dissolve In Water Alcohols can be considered derivatives of water (h 2 o; Mixing the two in any proportion generates a single solution. Whatever proportions you mix them in, you will get a single solution. In case of alcohols, just as it happens in case of many other biological molecules, the basic solubility rule that like. Alcohols of four or fewer carbon atoms. Why Does Alcohol Dissolve In Water.