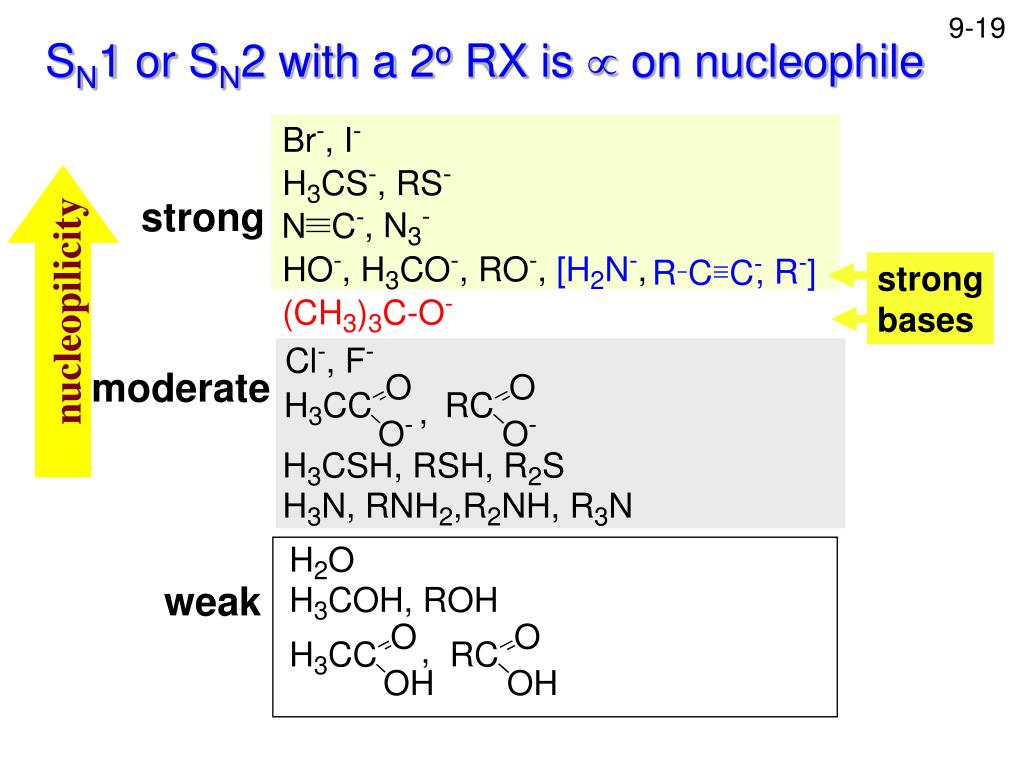
Base Nucleophile Definition . A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A nucleophile is a chemical species that donates an electron pair to an electrophile. A nucleophile can also be called a base when this. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. Here are some examples of lewis bases you’re probably. If they bond to a hydrogen atom, we call them.
from www.slideserve.com
All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). A nucleophile can also be called a base when this. If they bond to a hydrogen atom, we call them. A nucleophile is a chemical species that donates an electron pair to an electrophile. Here are some examples of lewis bases you’re probably. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a.
PPT Chapter 9 Nucleophilic Substitution & Elimination PowerPoint
Base Nucleophile Definition If they bond to a hydrogen atom, we call them. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A nucleophile is a chemical species that donates an electron pair to an electrophile. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. If they bond to a hydrogen atom, we call them. Here are some examples of lewis bases you’re probably. A nucleophile can also be called a base when this. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+).
From www.masterorganicchemistry.com
The Three Classes of Nucleophiles Master Organic Chemistry Base Nucleophile Definition Here are some examples of lewis bases you’re probably. If they bond to a hydrogen atom, we call them. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. A nucleophile can also be called a base when this. A “base” (or, “brønsted base”) is just the name we give to. Base Nucleophile Definition.
From collegedunia.com
Nucleophile Ambident Nucleophiles, Types & Mechanisms Base Nucleophile Definition A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A nucleophile can also be called a base when this. However, organic chemists usually refer to. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition If they bond to a hydrogen atom, we call them. A nucleophile can also be called a base when this. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. All. Base Nucleophile Definition.
From www.collegesearch.in
Difference Between Electrophile and Nucleophile Definitions, Examples Base Nucleophile Definition If they bond to a hydrogen atom, we call them. A nucleophile is a chemical species that donates an electron pair to an electrophile. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. A nucleophile can also be called a base when this. Here are some examples of lewis bases. Base Nucleophile Definition.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? — Master Organic Chemistry Base Nucleophile Definition A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond. Base Nucleophile Definition.
From www.slideserve.com
PPT 11. Reactions of Alkyl Halides Nucleophilic Substitutions and Base Nucleophile Definition A nucleophile can also be called a base when this. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. If they bond to a hydrogen atom, we call them. Here are some examples of lewis bases you’re probably. When the nucleophile donates a pair of electrons to a proton (h +). Base Nucleophile Definition.
From www.slideserve.com
PPT NUCLEOPHILICITY PowerPoint Presentation, free download ID6692309 Base Nucleophile Definition If they bond to a hydrogen atom, we call them. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). A nucleophile is a chemical species. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A “base”. Base Nucleophile Definition.
From mavink.com
Types Of Nucleophiles Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. A nucleophile is a chemical species that donates an electron pair to an electrophile. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. If they bond to a hydrogen atom, we call. Base Nucleophile Definition.
From www.chemistrysteps.com
Nucleophilic Substitution Reactions An Introduction Chemistry Steps Base Nucleophile Definition If they bond to a hydrogen atom, we call them. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A nucleophile is a chemical species that donates an electron pair to an electrophile. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Nucleophilicity vs. Basicity — Master Organic Chemistry Base Nucleophile Definition A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). Here are some examples of lewis bases you’re probably. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. However, organic chemists usually refer to a. Base Nucleophile Definition.
From openpress.usask.ca
11.5. Solving Problems Using Special Nucleophiles Introduction to Base Nucleophile Definition Here are some examples of lewis bases you’re probably. A nucleophile can also be called a base when this. If they bond to a hydrogen atom, we call them. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. All nucleophiles are brønsted bases — they donate a pair. Base Nucleophile Definition.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation ID207167 Base Nucleophile Definition Here are some examples of lewis bases you’re probably. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). When the nucleophile donates a pair of electrons to a. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base — Master Organic Base Nucleophile Definition However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A nucleophile can also be called a base when this. Here are some examples of lewis bases you’re probably. A nucleophile is a chemical species that donates an electron pair to an electrophile. If they bond to a hydrogen atom, we call. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). A nucleophile is a chemical species that donates an electron pair to an electrophile. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. Here are some examples of. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A nucleophile can also be called a base when this. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). When the nucleophile donates a pair of electrons to. Base Nucleophile Definition.
From www.aquaportail.com
Substitution nucléophile définition et explications Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. A nucleophile can also be called a base when this. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A “base” (or, “brønsted base”) is just the name we give to a. Base Nucleophile Definition.
From chem.libretexts.org
Nucleophiles Chemistry LibreTexts Base Nucleophile Definition A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). A nucleophile can also be called a base when this. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A nucleophile is a chemical species. Base Nucleophile Definition.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to. Base Nucleophile Definition.
From www.slideserve.com
PPT Part 3iv Substitution Reactions Nucleophile PowerPoint Base Nucleophile Definition Here are some examples of lewis bases you’re probably. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. If they bond to a hydrogen atom, we call. Base Nucleophile Definition.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Nucleophile Base Nucleophile Definition However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A “base”. Base Nucleophile Definition.
From allthedifferences.com
Base VS Nucleophile Understanding Important Facts All The Differences Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. A nucleophile can also be called a base when this. Here are some examples of lewis bases you’re probably. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. When the nucleophile donates. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. Here are some examples of lewis bases you’re probably. All nucleophiles are brønsted bases — they donate a pair of electrons. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition If they bond to a hydrogen atom, we call them. Here are some examples of lewis bases you’re probably. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another. Base Nucleophile Definition.
From www.chemistrylearner.com
Nucleophile Definition, Examples, and Strength Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. Here are some examples of lewis bases you’re probably. A nucleophile can also be called a base when this. A nucleophile is a. Base Nucleophile Definition.
From www.organicchemistrytutor.com
Nucleophiles and Electrophiles — Organic Chemistry Tutor Base Nucleophile Definition If they bond to a hydrogen atom, we call them. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. However, organic chemists usually refer to a lewis acid as. Base Nucleophile Definition.
From quizgorblimeys.z21.web.core.windows.net
What Is A Good Nucleophile Base Nucleophile Definition A nucleophile can also be called a base when this. A nucleophile is a chemical species that donates an electron pair to an electrophile. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a. Base Nucleophile Definition.
From www.masterorganicchemistry.com
The three types of nucleophiles you meet in organic chemistry — Master Base Nucleophile Definition Here are some examples of lewis bases you’re probably. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. A nucleophile can also be called a base when this. All nucleophiles. Base Nucleophile Definition.
From www.differencebetween.net
Difference Between Electrophile and Nucleophile Difference Between Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. If they bond to a hydrogen atom, we call them. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. Here are some examples of lewis bases you’re probably. A nucleophile is a. Base Nucleophile Definition.
From chemistry.stackexchange.com
organic chemistry Definition of nucleophile Chemistry Stack Exchange Base Nucleophile Definition A nucleophile can also be called a base when this. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). All nucleophiles are brønsted bases — they donate a. Base Nucleophile Definition.
From www.slideserve.com
PPT Chapter 9 Nucleophilic Substitution & Elimination PowerPoint Base Nucleophile Definition When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition A nucleophile can also be called a base when this. A nucleophile is a chemical species that donates an electron pair to an electrophile. However, organic chemists usually refer to a lewis acid as an electrophile (which is electron poor), and a. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a. Base Nucleophile Definition.
From www.science-revision.co.uk
Nucleophilic substitution examples Base Nucleophile Definition If they bond to a hydrogen atom, we call them. A nucleophile can also be called a base when this. When the nucleophile donates a pair of electrons to a proton (h +) it’s called a brønsted base, or simply, “base”. All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom.. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom. If they bond to a hydrogen atom, we call them. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). However, organic chemists usually refer to a lewis. Base Nucleophile Definition.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Base Nucleophile Definition Here are some examples of lewis bases you’re probably. A nucleophile can also be called a base when this. A “base” (or, “brønsted base”) is just the name we give to a nucleophile when it’s forming a bond to a proton (h+). All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another. Base Nucleophile Definition.