Examples Of Water Polar Solvent . Water has many properties that are critical to maintaining life. Why water makes a good solvent, and what kinds of molecules dissolve best in it. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Hydrogen bonds allow ions and other polar molecules to. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. There are many examples (too many to list) where a polar protic solvent such as water,. Which substances should dissolve in water? These types of solvents are by far the most likely to participate in reactions. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Example \(\pageindex{2}\) water is considered a polar solvent. It is a polar molecule, allowing for the formation of hydrogen bonds.
from socratic.org
Which substances should dissolve in water? Why water makes a good solvent, and what kinds of molecules dissolve best in it. Hydrogen bonds allow ions and other polar molecules to. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water has many properties that are critical to maintaining life. It is a polar molecule, allowing for the formation of hydrogen bonds. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. These types of solvents are by far the most likely to participate in reactions. There are many examples (too many to list) where a polar protic solvent such as water,.
What kinds of molecules are polar? + Example
Examples Of Water Polar Solvent Example \(\pageindex{2}\) water is considered a polar solvent. Water has many properties that are critical to maintaining life. Which substances should dissolve in water? Example \(\pageindex{2}\) water is considered a polar solvent. Hydrogen bonds allow ions and other polar molecules to. Why water makes a good solvent, and what kinds of molecules dissolve best in it. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. These types of solvents are by far the most likely to participate in reactions. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. There are many examples (too many to list) where a polar protic solvent such as water,. It is a polar molecule, allowing for the formation of hydrogen bonds. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Examples Of Water Polar Solvent There are many examples (too many to list) where a polar protic solvent such as water,. These types of solvents are by far the most likely to participate in reactions. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water has many properties that are critical to maintaining. Examples Of Water Polar Solvent.
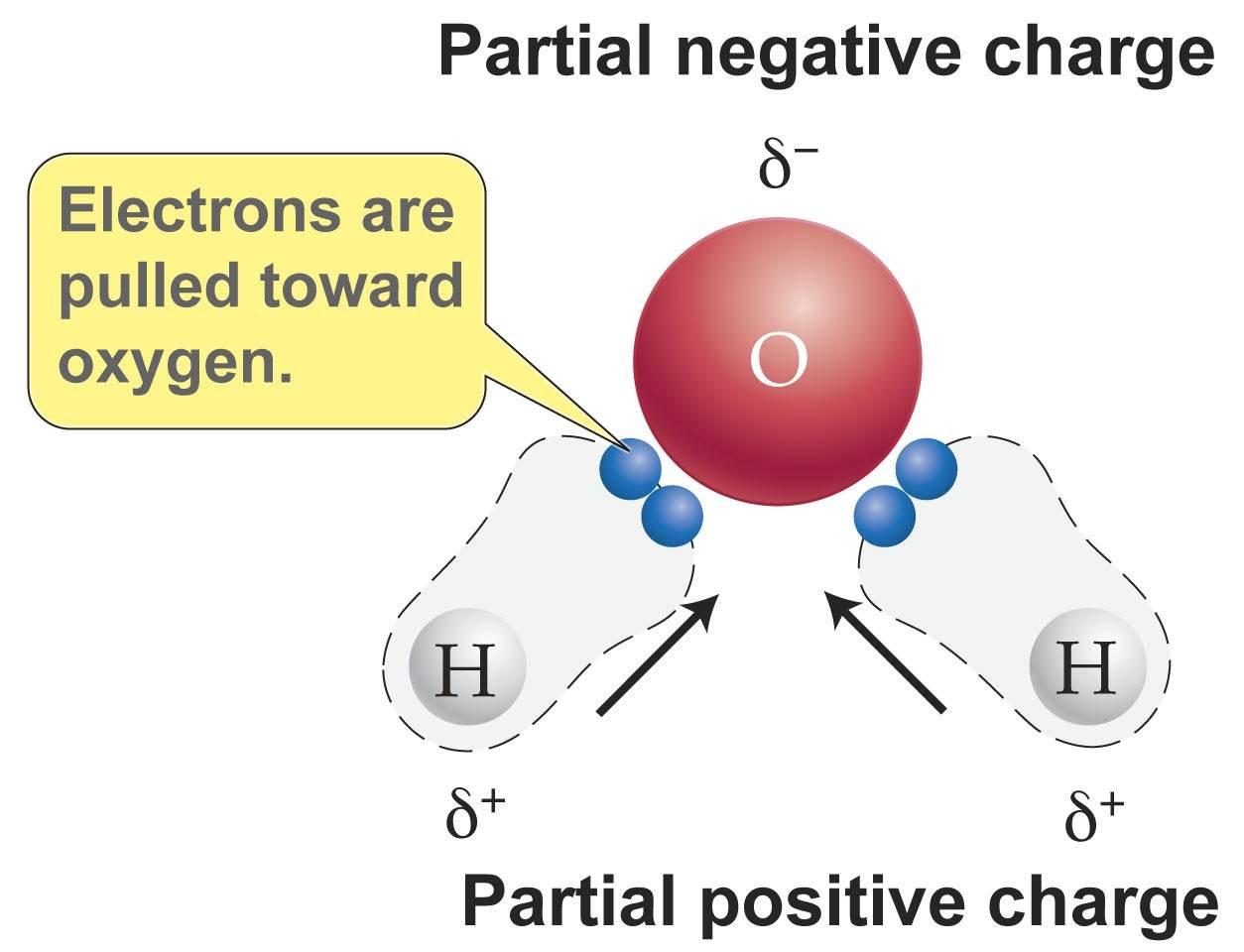
From studylistboelter.z13.web.core.windows.net
Explain How Water Is The Universal Solvent Examples Of Water Polar Solvent There are many examples (too many to list) where a polar protic solvent such as water,. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. These types of solvents are by far the most likely to. Examples Of Water Polar Solvent.
From slideplayer.com
UNIT 6 Solution Chemistry. ppt download Examples Of Water Polar Solvent It is a polar molecule, allowing for the formation of hydrogen bonds. Water has many properties that are critical to maintaining life. There are many examples (too many to list) where a polar protic solvent such as water,. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water is a polar molecule because of its. Examples Of Water Polar Solvent.
From webapi.bu.edu
💌 Water universal solvent examples. Water Molecule & Polarity. 20221024 Examples Of Water Polar Solvent But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. These types of solvents are by far the most likely to participate in reactions. There are many examples (too many to list) where a polar protic solvent. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Biological Molecules PowerPoint Presentation, free download ID Examples Of Water Polar Solvent Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Why water makes a good solvent, and what kinds of molecules dissolve best in it. It is a polar molecule, allowing for the formation of hydrogen bonds. Water is a polar molecule because of its bent geometry and the. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free Examples Of Water Polar Solvent But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Hydrogen bonds allow ions and other polar molecules to. There are many examples (too many to list) where a polar protic solvent such as water,. It is a polar molecule, allowing for the formation of hydrogen bonds. Example \(\pageindex{2}\) water is considered a polar solvent. Water, which not. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 Examples Of Water Polar Solvent Which substances should dissolve in water? These types of solvents are by far the most likely to participate in reactions. Water has many properties that are critical to maintaining life. Example \(\pageindex{2}\) water is considered a polar solvent. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water. Examples Of Water Polar Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Examples Of Water Polar Solvent Why water makes a good solvent, and what kinds of molecules dissolve best in it. These types of solvents are by far the most likely to participate in reactions. Which substances should dissolve in water? But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Hydrogen bonds allow ions and other polar molecules to. There are many examples. Examples Of Water Polar Solvent.
From mungfali.com
Polar Water Molecule Diagram Examples Of Water Polar Solvent Example \(\pageindex{2}\) water is considered a polar solvent. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Hydrogen bonds allow ions and other polar molecules to. It is a polar molecule, allowing for the formation. Examples Of Water Polar Solvent.
From mavink.com
Polarity Chart Of Solvents Examples Of Water Polar Solvent Example \(\pageindex{2}\) water is considered a polar solvent. Which substances should dissolve in water? Hydrogen bonds allow ions and other polar molecules to. Why water makes a good solvent, and what kinds of molecules dissolve best in it. It is a polar molecule, allowing for the formation of hydrogen bonds. Water, which not only dissolves many compounds but also dissolves. Examples Of Water Polar Solvent.
From exyytwowd.blob.core.windows.net
Examples Of Polar Solvent In Chemistry at Martin Harris blog Examples Of Water Polar Solvent Which substances should dissolve in water? But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. It is a polar molecule, allowing for the formation of hydrogen bonds. Water has many properties that are critical to maintaining life. Hydrogen bonds allow ions and other. Examples Of Water Polar Solvent.
From study.com
Water Molecule Properties, Structure & Polarity Lesson Examples Of Water Polar Solvent Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. There are many examples (too many to list) where a polar protic solvent such as water,. But, it isn’t. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID375520 Examples Of Water Polar Solvent These types of solvents are by far the most likely to participate in reactions. Which substances should dissolve in water? It is a polar molecule, allowing for the formation of hydrogen bonds. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Why water makes a good solvent, and what kinds of molecules dissolve best in. Examples Of Water Polar Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Examples Of Water Polar Solvent Water has many properties that are critical to maintaining life. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Why water makes a good solvent, and what kinds of molecules dissolve best in it. Example \(\pageindex{2}\) water is considered a polar solvent. It is a polar molecule, allowing for the formation of hydrogen bonds. Water, which not. Examples Of Water Polar Solvent.
From socratic.org
What kinds of molecules are polar? + Example Examples Of Water Polar Solvent Hydrogen bonds allow ions and other polar molecules to. Which substances should dissolve in water? There are many examples (too many to list) where a polar protic solvent such as water,. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Example \(\pageindex{2}\) water is considered a polar solvent.. Examples Of Water Polar Solvent.
From biostudizz.weebly.com
WATER CHEMISTRY to Bio Stud... Examples Of Water Polar Solvent Example \(\pageindex{2}\) water is considered a polar solvent. Hydrogen bonds allow ions and other polar molecules to. There are many examples (too many to list) where a polar protic solvent such as water,. Water has many properties that are critical to maintaining life. Which substances should dissolve in water? Why water makes a good solvent, and what kinds of molecules. Examples Of Water Polar Solvent.
From sciencenotes.org
Why Is Water Called the Universal Solvent? Examples Of Water Polar Solvent It is a polar molecule, allowing for the formation of hydrogen bonds. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water has many properties that are critical to maintaining life. There are many examples (too many to list) where a polar protic solvent such as water,. Example \(\pageindex{2}\) water is considered a polar solvent. Water is. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 Examples Of Water Polar Solvent Hydrogen bonds allow ions and other polar molecules to. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Example \(\pageindex{2}\) water is considered a polar solvent. These types of solvents are by far the most likely to participate in reactions. Water has many properties that are critical to maintaining. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Examples Of Water Polar Solvent Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. There are many examples (too many to list) where a polar protic solvent such as water,. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. But, it isn’t. Examples Of Water Polar Solvent.
From www.youtube.com
Science, Grade 9 Water Polarity & Universal Solvent Lido Learning Examples Of Water Polar Solvent There are many examples (too many to list) where a polar protic solvent such as water,. Water has many properties that are critical to maintaining life. Which substances should dissolve in water? But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water, which. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Examples Of Water Polar Solvent It is a polar molecule, allowing for the formation of hydrogen bonds. Which substances should dissolve in water? Hydrogen bonds allow ions and other polar molecules to. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water has many properties that are critical to maintaining life. Water, which not only dissolves many compounds but also dissolves more. Examples Of Water Polar Solvent.
From exyytwowd.blob.core.windows.net
Examples Of Polar Solvent In Chemistry at Martin Harris blog Examples Of Water Polar Solvent Which substances should dissolve in water? Why water makes a good solvent, and what kinds of molecules dissolve best in it. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. It is a polar molecule, allowing for the formation of hydrogen bonds. But, it isn’t a universal solvent. Examples Of Water Polar Solvent.
From www.youtube.com
Chapter 08 05 Water A Polar Solvent YouTube Examples Of Water Polar Solvent Why water makes a good solvent, and what kinds of molecules dissolve best in it. Hydrogen bonds allow ions and other polar molecules to. There are many examples (too many to list) where a polar protic solvent such as water,. It is a polar molecule, allowing for the formation of hydrogen bonds. Water dissolves polar molecules, including salts, sugars, many. Examples Of Water Polar Solvent.
From www.chemistryworld.com
Polar solvents promote halogen bonds over hydrogen ones Research Examples Of Water Polar Solvent Why water makes a good solvent, and what kinds of molecules dissolve best in it. There are many examples (too many to list) where a polar protic solvent such as water,. It is a polar molecule, allowing for the formation of hydrogen bonds. These types of solvents are by far the most likely to participate in reactions. Water has many. Examples Of Water Polar Solvent.
From slideplayer.com
Solutions. ppt download Examples Of Water Polar Solvent It is a polar molecule, allowing for the formation of hydrogen bonds. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Example \(\pageindex{2}\) water is considered a polar solvent. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Why water makes a good solvent, and. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Examples Of Water Polar Solvent These types of solvents are by far the most likely to participate in reactions. Water has many properties that are critical to maintaining life. Hydrogen bonds allow ions and other polar molecules to. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. There are many examples (too many to list) where a polar protic solvent. Examples Of Water Polar Solvent.
From www.pinterest.com
Why Is Water a Polar Molecule? Water molecule, Molecular geometry Examples Of Water Polar Solvent These types of solvents are by far the most likely to participate in reactions. It is a polar molecule, allowing for the formation of hydrogen bonds. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. There are many examples (too many to list) where a polar protic solvent. Examples Of Water Polar Solvent.
From tutors.com
What is a solvent? Definition & Examples (Video) Examples Of Water Polar Solvent Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Which substances should dissolve in water? Why water makes a good solvent, and what kinds of molecules dissolve best in it. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Hydrogen bonds allow ions and. Examples Of Water Polar Solvent.
From exyytwowd.blob.core.windows.net
Examples Of Polar Solvent In Chemistry at Martin Harris blog Examples Of Water Polar Solvent Why water makes a good solvent, and what kinds of molecules dissolve best in it. Water has many properties that are critical to maintaining life. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Example \(\pageindex{2}\) water is considered a polar solvent. Water, which not only dissolves many compounds but also dissolves more substances than. Examples Of Water Polar Solvent.
From studylib.net
Water The Universal Solvent Examples Of Water Polar Solvent There are many examples (too many to list) where a polar protic solvent such as water,. It is a polar molecule, allowing for the formation of hydrogen bonds. Water has many properties that are critical to maintaining life. Example \(\pageindex{2}\) water is considered a polar solvent. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna.. Examples Of Water Polar Solvent.
From webapi.bu.edu
💌 Water universal solvent examples. Water Molecule & Polarity. 20221024 Examples Of Water Polar Solvent Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. These types of solvents are by far the most likely to participate in reactions. Which substances should dissolve in water? Hydrogen bonds allow ions and other polar molecules to. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Examples Of Water Polar Solvent.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Examples Of Water Polar Solvent These types of solvents are by far the most likely to participate in reactions. Which substances should dissolve in water? Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Hydrogen bonds allow ions and other polar molecules. Examples Of Water Polar Solvent.
From www.youtube.com
Polar Protic Solvents and Polar Aprotic Solvents For SN1 & SN2 Examples Of Water Polar Solvent Which substances should dissolve in water? But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. These types of solvents are by far the most likely to participate in reactions. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. It is a polar molecule, allowing for the formation of hydrogen bonds. There are. Examples Of Water Polar Solvent.
From www.expii.com
Good Solvent (Water) — Properties & Examples Expii Examples Of Water Polar Solvent Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Example \(\pageindex{2}\) water is considered a polar solvent. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Why water makes a good solvent,. Examples Of Water Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3746599 Examples Of Water Polar Solvent Why water makes a good solvent, and what kinds of molecules dissolve best in it. There are many examples (too many to list) where a polar protic solvent such as water,. These types of solvents are by far the most likely to participate in reactions. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water dissolves polar. Examples Of Water Polar Solvent.