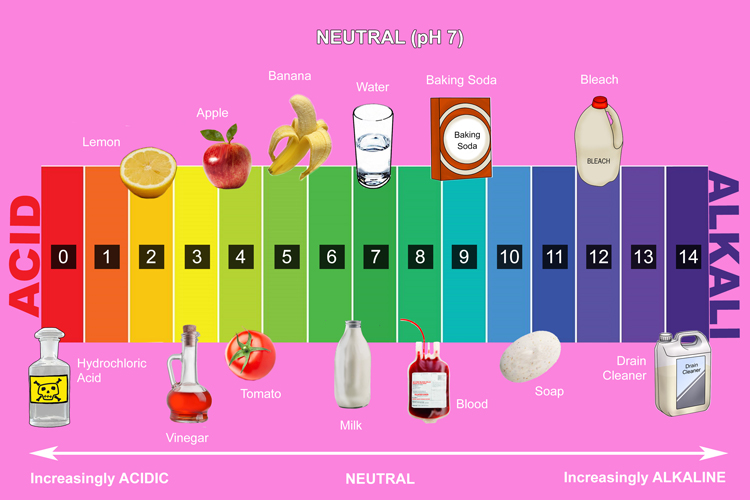
How Do Natural Ph Indicators Work . Most ph indicators are weak acids or weak bases. Hind + h 2 o ⇌ h 3 o + + ind −. The reaction changes the color of the indicator molecule. There are many common household products and garden plants that can be used as ph indicators. They are themselves weak acids and bases. They dissociate according to the general chemical reaction: The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. How do ph indicators work? In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Many of these natural ph indicators exhibit a broad range of colors. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. If an indicator is a weak.
from mammothmemory.net
They dissociate according to the general chemical reaction: Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. Hind + h 2 o ⇌ h 3 o + + ind −. How do ph indicators work? They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are substances whose solutions change color due to changes in ph. There are many common household products and garden plants that can be used as ph indicators. Many of these natural ph indicators exhibit a broad range of colors. Most ph indicators are weak acids or weak bases.
There are 2 indicators litmus paper or universal indicator
How Do Natural Ph Indicators Work If an indicator is a weak. How do ph indicators work? They dissociate according to the general chemical reaction: The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. There are many common household products and garden plants that can be used as ph indicators. Many of these natural ph indicators exhibit a broad range of colors. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Hind + h 2 o ⇌ h 3 o + + ind −. They are themselves weak acids and bases. Most ph indicators are weak acids or weak bases. Indicators are substances whose solutions change color due to changes in ph. If an indicator is a weak. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. The reaction changes the color of the indicator molecule.
From discover.hubpages.com
What is Universal Indicator and How To Use it HubPages How Do Natural Ph Indicators Work Many of these natural ph indicators exhibit a broad range of colors. The reaction changes the color of the indicator molecule. Indicators are substances whose solutions change color due to changes in ph. They are themselves weak acids and bases. Hind + h 2 o ⇌ h 3 o + + ind −. How do ph indicators work? In more. How Do Natural Ph Indicators Work.
From www.flinnsci.com
360 Science Introduction to pH Scale and Indicators How Do Natural Ph Indicators Work Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. Hind + h 2 o ⇌. How Do Natural Ph Indicators Work.
From www.chemedx.org
Making Natural Acid Base Indicators Chemical Education Xchange How Do Natural Ph Indicators Work How do ph indicators work? Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Many of these natural ph indicators exhibit a broad range of colors. The reaction changes the color of the indicator molecule. There are many common household products and garden. How Do Natural Ph Indicators Work.
From ar.inspiredpencil.com
Ph Scale Examples For Kids How Do Natural Ph Indicators Work There are many common household products and garden plants that can be used as ph indicators. Hind + h 2 o ⇌ h 3 o + + ind −. The reaction changes the color of the indicator molecule. They are themselves weak acids and bases. The principle behind the function of an indicator is that it reacts with water to. How Do Natural Ph Indicators Work.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ How Do Natural Ph Indicators Work They are themselves weak acids and bases. There are many common household products and garden plants that can be used as ph indicators. If an indicator is a weak. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. They are usually weak acids. How Do Natural Ph Indicators Work.
From www.flinnsci.com
pH Indicator Chart How Do Natural Ph Indicators Work Most ph indicators are weak acids or weak bases. Many of these natural ph indicators exhibit a broad range of colors. If an indicator is a weak. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. There are many common household products and garden plants. How Do Natural Ph Indicators Work.
From www.science-sparks.com
How to make pH indicator with a poinsettia How Do Natural Ph Indicators Work There are many common household products and garden plants that can be used as ph indicators. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Most ph indicators are weak acids or weak bases. Indicators are substances whose solutions change color due to. How Do Natural Ph Indicators Work.
From www.science-sparks.com
How to make a red cabbage pH indicator Chemistry for Kids How Do Natural Ph Indicators Work They are themselves weak acids and bases. Hind + h 2 o ⇌ h 3 o + + ind −. If an indicator is a weak. Most ph indicators are weak acids or weak bases. The reaction changes the color of the indicator molecule. Indicators are substances whose solutions change color due to changes in ph. How do ph indicators. How Do Natural Ph Indicators Work.
From testbook.com
[Solved] An aqueous solution with pH = 0 is How Do Natural Ph Indicators Work Most ph indicators are weak acids or weak bases. They dissociate according to the general chemical reaction: Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. There are many common household products and garden plants that can be used as ph indicators. Indicators. How Do Natural Ph Indicators Work.
From www.youtube.com
Universal Indicators and the pH scale YouTube How Do Natural Ph Indicators Work There are many common household products and garden plants that can be used as ph indicators. If an indicator is a weak. The reaction changes the color of the indicator molecule. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Most ph indicators. How Do Natural Ph Indicators Work.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Do Natural Ph Indicators Work They dissociate according to the general chemical reaction: How do ph indicators work? In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Hind + h 2 o ⇌ h 3 o + + ind −. Indicators are substances whose solutions change color due to. How Do Natural Ph Indicators Work.
From descargas.intef.es
Elaboración de un indicador natural de pH La madre de todas las masas How Do Natural Ph Indicators Work How do ph indicators work? Many of these natural ph indicators exhibit a broad range of colors. If an indicator is a weak. Indicators are substances whose solutions change color due to changes in ph. The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h. How Do Natural Ph Indicators Work.
From www.dreamstime.com
PH Scale Indicator Chart Diagram Acidic Alkaline Measure. PH Analysis How Do Natural Ph Indicators Work In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. They dissociate according to the general chemical reaction: How do ph indicators work? Many of these natural ph indicators exhibit a broad range of colors. Indicators are substances whose solutions change color due to changes. How Do Natural Ph Indicators Work.
From www.thoughtco.com
What Is a pH Indicator? Definition and Examples How Do Natural Ph Indicators Work They dissociate according to the general chemical reaction: Hind + h 2 o ⇌ h 3 o + + ind −. They are themselves weak acids and bases. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. If an indicator is a weak.. How Do Natural Ph Indicators Work.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Do Natural Ph Indicators Work In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. The reaction changes the color of the indicator molecule. Indicators are substances whose solutions change color due to changes in ph. There are many common household products and garden plants that can be used as. How Do Natural Ph Indicators Work.
From rosieresearch.com
Natural pH Indicators Make a pH indicator using beetroot or cabbage! How Do Natural Ph Indicators Work Many of these natural ph indicators exhibit a broad range of colors. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. How do ph indicators work? Most ph indicators are weak acids or weak bases. There are many common household products and garden plants. How Do Natural Ph Indicators Work.
From www.slideshare.net
ppt_acids and bases How Do Natural Ph Indicators Work In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Many of these natural ph indicators exhibit a broad range of colors. They are themselves weak acids and bases. They are usually weak acids or bases, but their conjugate base or acid forms have different. How Do Natural Ph Indicators Work.
From www.youtube.com
Making natural pH indicator at home! YouTube How Do Natural Ph Indicators Work The reaction changes the color of the indicator molecule. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. There are many common household products and garden plants that can be used as ph indicators. Most ph indicators are weak acids or weak bases. Ph indicators. How Do Natural Ph Indicators Work.
From chemistry-acidsandbases.weebly.com
pH Scale and pH Indicators How Do Natural Ph Indicators Work If an indicator is a weak. Indicators are substances whose solutions change color due to changes in ph. Many of these natural ph indicators exhibit a broad range of colors. They dissociate according to the general chemical reaction: The reaction changes the color of the indicator molecule. Hind + h 2 o ⇌ h 3 o + + ind −.. How Do Natural Ph Indicators Work.
From www.slideserve.com
PPT Making our own pH indicators PowerPoint Presentation, free How Do Natural Ph Indicators Work Most ph indicators are weak acids or weak bases. How do ph indicators work? Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences. How Do Natural Ph Indicators Work.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest How Do Natural Ph Indicators Work The reaction changes the color of the indicator molecule. The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. They dissociate according to the general chemical reaction: Ph indicators are weak acids that exist as natural dyes and indicate the concentration of. How Do Natural Ph Indicators Work.
From www.vectorstock.com
Ph scale universal indicator ph color chart Vector Image How Do Natural Ph Indicators Work There are many common household products and garden plants that can be used as ph indicators. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. How do ph indicators work? Ph indicators are weak acids that exist as natural dyes and indicate the concentration. How Do Natural Ph Indicators Work.
From www.freepik.es
Imágenes de Escala Ph Descarga gratuita en Freepik How Do Natural Ph Indicators Work The reaction changes the color of the indicator molecule. If an indicator is a weak. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. How do ph indicators work? They are themselves weak acids and bases. There are many common household products and. How Do Natural Ph Indicators Work.
From www.scienceinschool.org
Little wonder pH experiments the microscale way Science in School How Do Natural Ph Indicators Work The reaction changes the color of the indicator molecule. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. There are many common household products and garden plants that can be used as ph indicators. They dissociate according to the general chemical reaction: Ph indicators are. How Do Natural Ph Indicators Work.
From www.vedantu.com
Defining pH Indicator and Understanding its Importance in Life How Do Natural Ph Indicators Work In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Most ph indicators are weak acids or weak bases. Many of these natural ph indicators exhibit a broad range of colors. How do ph indicators work? If an indicator is a weak. Hind + h. How Do Natural Ph Indicators Work.
From www.youtube.com
A Simple Guide to the pH Scale and Indicators YouTube How Do Natural Ph Indicators Work They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Many of these natural ph indicators exhibit a broad range of. How Do Natural Ph Indicators Work.
From soapsmithnotes.blogspot.com
Soapsmith's Notes The pH of hair, skin and products (and why it matters!) How Do Natural Ph Indicators Work Hind + h 2 o ⇌ h 3 o + + ind −. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. The reaction changes the color of the indicator molecule. Many of these natural ph indicators exhibit a broad range of colors.. How Do Natural Ph Indicators Work.
From www.tes.com
Indicators and pH Teaching Resources How Do Natural Ph Indicators Work Hind + h 2 o ⇌ h 3 o + + ind −. Indicators are substances whose solutions change color due to changes in ph. They dissociate according to the general chemical reaction: If an indicator is a weak. There are many common household products and garden plants that can be used as ph indicators. The principle behind the function. How Do Natural Ph Indicators Work.
From www.vectorstock.com
Ph scale universal indicator color chart Vector Image How Do Natural Ph Indicators Work The reaction changes the color of the indicator molecule. The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. They are themselves weak acids and bases. Most ph indicators are weak acids or weak bases. Many of these natural ph indicators exhibit. How Do Natural Ph Indicators Work.
From mammothmemory.net
There are 2 indicators litmus paper or universal indicator How Do Natural Ph Indicators Work Most ph indicators are weak acids or weak bases. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. They dissociate according to the general chemical reaction: In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph >. How Do Natural Ph Indicators Work.
From rosieresearch.com
Natural pH Indicators Make a pH indicator using beetroot or cabbage! How Do Natural Ph Indicators Work They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. Indicators are substances whose solutions change color due. How Do Natural Ph Indicators Work.
From www.science-sparks.com
What is the pH Scale? How Do Natural Ph Indicators Work If an indicator is a weak. How do ph indicators work? The reaction changes the color of the indicator molecule. They dissociate according to the general chemical reaction: They are themselves weak acids and bases. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink.. How Do Natural Ph Indicators Work.
From rosieresearch.com
Natural pH Indicators Make a pH indicator using beetroot or cabbage! How Do Natural Ph Indicators Work Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. There are many common household products and garden plants that. How Do Natural Ph Indicators Work.
From www.chegg.com
Solved 1. Below is a list of common pH indicators and their How Do Natural Ph Indicators Work Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. They are themselves weak acids and bases. Hind + h 2 o ⇌ h 3 o + + ind −. There are many common household products and garden plants that can be used as. How Do Natural Ph Indicators Work.
From learn.careers360.com
Make a list of various natural and synthetic indicators and write How Do Natural Ph Indicators Work Most ph indicators are weak acids or weak bases. How do ph indicators work? The principle behind the function of an indicator is that it reacts with water to form the hydrogen cation h + or hydronium ion h 3 o +. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\. How Do Natural Ph Indicators Work.