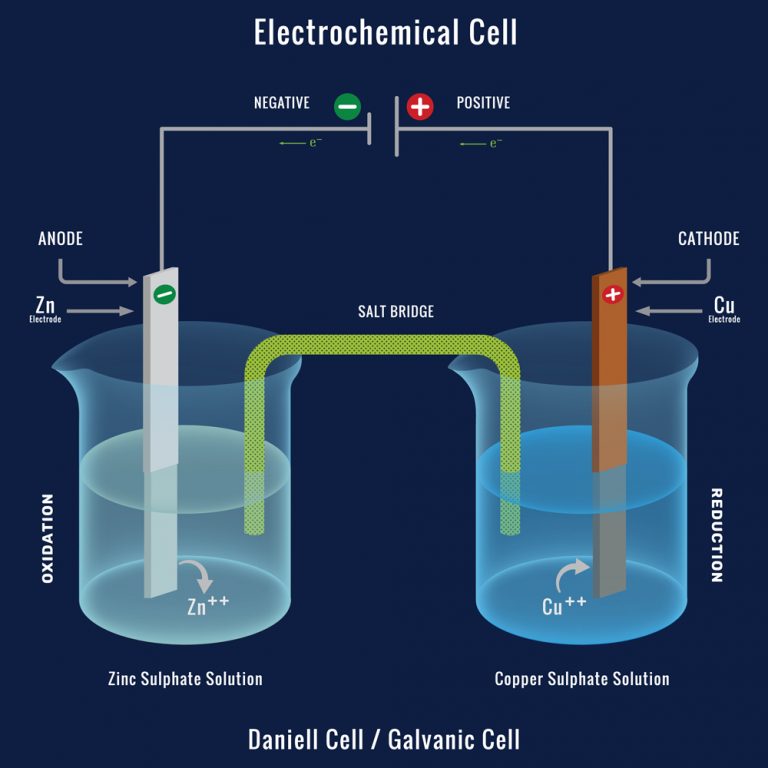
A Galvanic Cell Composed Of . Describe the function of a galvanic cell and its components; A galvanic cell converts a chemical reaction into electricity. Describe the basic components of galvanic cells. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. It is used to supply electrical current through a redox reaction to the transfer of electrons. Galvanic cells were first described in. Use cell notation to describe galvanic cells. The electrochemical cell type is a galvanic cell. Use cell notation to symbolize the composition and construction of galvanic cells A battery is a galvanic cell.
from www.scienceabc.com
Describe the basic components of galvanic cells. A galvanic cell converts a chemical reaction into electricity. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The electrochemical cell type is a galvanic cell. It is used to supply electrical current through a redox reaction to the transfer of electrons. Galvanic cells were first described in. A battery is a galvanic cell. Describe the function of a galvanic cell and its components; Use cell notation to describe galvanic cells.
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC
A Galvanic Cell Composed Of It is used to supply electrical current through a redox reaction to the transfer of electrons. It is used to supply electrical current through a redox reaction to the transfer of electrons. A galvanic cell converts a chemical reaction into electricity. Use cell notation to symbolize the composition and construction of galvanic cells Describe the basic components of galvanic cells. Galvanic cells were first described in. A battery is a galvanic cell. The electrochemical cell type is a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Describe the function of a galvanic cell and its components; Use cell notation to describe galvanic cells. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC A Galvanic Cell Composed Of Describe the basic components of galvanic cells. It is used to supply electrical current through a redox reaction to the transfer of electrons. Galvanic cells were first described in. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Describe the function of a galvanic cell and its components; A galvanic. A Galvanic Cell Composed Of.
From www.pinterest.com
Galvanic cell. Galvanic cell, Chemistry basics, Electrochemical cell A Galvanic Cell Composed Of The electrochemical cell type is a galvanic cell. Galvanic cells were first described in. It is used to supply electrical current through a redox reaction to the transfer of electrons. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A galvanic cell is an example of how to use simple reactions between. A Galvanic Cell Composed Of.
From courses.lumenlearning.com
Galvanic Cells General Chemistry A Galvanic Cell Composed Of Describe the function of a galvanic cell and its components; A battery is a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Galvanic cells were first described in. Use cell notation to describe galvanic cells. The electrochemical cell type is a galvanic cell. Describe the basic components of galvanic. A Galvanic Cell Composed Of.
From www.shutterstock.com
Diagrama de células electroquímicas. Célula galvánica vector de stock A Galvanic Cell Composed Of A galvanic cell converts a chemical reaction into electricity. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Describe the function of a galvanic cell and its components; A battery is a galvanic cell. Use cell notation to symbolize the composition and construction of galvanic cells Galvanic cells were first. A Galvanic Cell Composed Of.
From school.careers360.com
galvanic cell Overview, Structure, Properties & Uses A Galvanic Cell Composed Of A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. A battery is a galvanic cell. Use cell notation to describe galvanic cells. Use cell notation to symbolize the composition and construction of galvanic cells A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure. A Galvanic Cell Composed Of.
From www.chegg.com
Solved Sketch a diagram of a galvanic cell represented by A Galvanic Cell Composed Of Use cell notation to symbolize the composition and construction of galvanic cells A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. A galvanic cell converts a chemical reaction into electricity. Describe the function of a galvanic cell and its components; Describe the basic components of galvanic cells. Use cell notation. A Galvanic Cell Composed Of.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 A Galvanic Cell Composed Of Use cell notation to describe galvanic cells. Galvanic cells were first described in. The electrochemical cell type is a galvanic cell. Describe the function of a galvanic cell and its components; Describe the basic components of galvanic cells. A battery is a galvanic cell. A galvanic cell converts a chemical reaction into electricity. A galvanic cell is an example of. A Galvanic Cell Composed Of.
From www.shutterstock.com
Schematic Galvanic Cell Stock Vector (Royalty Free) 1980381947 A Galvanic Cell Composed Of Use cell notation to symbolize the composition and construction of galvanic cells Describe the function of a galvanic cell and its components; Describe the basic components of galvanic cells. Galvanic cells were first described in. Use cell notation to describe galvanic cells. A galvanic cell is an example of how to use simple reactions between a few elements to harness. A Galvanic Cell Composed Of.
From ar.inspiredpencil.com
Galvanic Cell Labeled A Galvanic Cell Composed Of Galvanic cells were first described in. Use cell notation to describe galvanic cells. The electrochemical cell type is a galvanic cell. A battery is a galvanic cell. Describe the function of a galvanic cell and its components; A galvanic cell converts a chemical reaction into electricity. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted. A Galvanic Cell Composed Of.
From www.collegesearch.in
Galvanic Cells Definitions, Examples, How They are Created, Principles A Galvanic Cell Composed Of The electrochemical cell type is a galvanic cell. It is used to supply electrical current through a redox reaction to the transfer of electrons. A galvanic cell converts a chemical reaction into electricity. Use cell notation to describe galvanic cells. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A galvanic cell. A Galvanic Cell Composed Of.
From users.highland.edu
Commercial Galvanic Cells A Galvanic Cell Composed Of A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Use cell notation to describe galvanic cells. It is used to supply electrical current through a redox reaction to the transfer of electrons. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A. A Galvanic Cell Composed Of.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle A Galvanic Cell Composed Of A galvanic cell converts a chemical reaction into electricity. Describe the basic components of galvanic cells. The electrochemical cell type is a galvanic cell. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Galvanic cells were first described in. Use cell notation to symbolize the composition and construction of galvanic. A Galvanic Cell Composed Of.
From www.studypool.com
SOLUTION Galvanic cell their representation Studypool A Galvanic Cell Composed Of A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Galvanic cells were first described in. A battery is a galvanic cell. Use cell notation to describe galvanic cells. A galvanic cell converts a chemical reaction into electricity. It is used to supply electrical current through a redox reaction to the transfer of. A Galvanic Cell Composed Of.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and A Galvanic Cell Composed Of A galvanic cell converts a chemical reaction into electricity. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. It is used to supply electrical current through a redox reaction to the transfer of electrons. A battery is a galvanic cell. Use cell notation to symbolize the composition and construction of galvanic cells. A Galvanic Cell Composed Of.
From www.slideserve.com
PPT Galvanic Cells Converting chemical energy to electrical energy A Galvanic Cell Composed Of A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Use cell notation to describe galvanic cells. The electrochemical cell type is a galvanic cell. Describe the function of a galvanic cell and its components; A battery is a galvanic cell. A galvanic cell converts a chemical reaction into electricity. Describe. A Galvanic Cell Composed Of.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner A Galvanic Cell Composed Of A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A battery is a galvanic cell. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Use cell notation to describe galvanic cells. Use cell notation to symbolize the composition and construction of galvanic. A Galvanic Cell Composed Of.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo A Galvanic Cell Composed Of A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Describe the function of a galvanic cell and its components; It is used to supply electrical current through a redox reaction to the transfer. A Galvanic Cell Composed Of.
From www.youtube.com
Galvanic Cell Example Daniell Cell YouTube A Galvanic Cell Composed Of Describe the basic components of galvanic cells. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. A battery is a galvanic cell. Use cell notation to describe galvanic cells. The electrochemical cell type is a galvanic cell. Galvanic cells were first described in. A galvanic cell based on the spontaneous. A Galvanic Cell Composed Of.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Galvanic Cell Composed Of Use cell notation to describe galvanic cells. The electrochemical cell type is a galvanic cell. Galvanic cells were first described in. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Use cell notation. A Galvanic Cell Composed Of.
From www.youtube.com
Galvanic Cells Explained // Preliminary HSC Chemistry YouTube A Galvanic Cell Composed Of It is used to supply electrical current through a redox reaction to the transfer of electrons. Describe the function of a galvanic cell and its components; A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Describe the basic components of galvanic cells. Use cell notation to describe galvanic cells. A galvanic cell. A Galvanic Cell Composed Of.
From chem.eduinsightful.com
Galvanic Cells Decoded Igniting a Spark of Knowledge A Galvanic Cell Composed Of Describe the basic components of galvanic cells. Use cell notation to describe galvanic cells. Describe the function of a galvanic cell and its components; Galvanic cells were first described in. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Use cell notation to symbolize the composition and construction of galvanic. A Galvanic Cell Composed Of.
From www.chemistrysources.com
الخلية الجلفانية Galvanic Cell مصادر الكيمياء A Galvanic Cell Composed Of Describe the basic components of galvanic cells. Describe the function of a galvanic cell and its components; Use cell notation to describe galvanic cells. Galvanic cells were first described in. Use cell notation to symbolize the composition and construction of galvanic cells The electrochemical cell type is a galvanic cell. A galvanic cell based on the spontaneous reaction between copper. A Galvanic Cell Composed Of.
From www.clutchprep.com
Galvanic Cell Chemistry Video Clutch Prep A Galvanic Cell Composed Of Describe the function of a galvanic cell and its components; Use cell notation to describe galvanic cells. It is used to supply electrical current through a redox reaction to the transfer of electrons. A battery is a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A galvanic cell is. A Galvanic Cell Composed Of.
From www.toppr.com
What are galvanic cells? Explain the construction and working of A Galvanic Cell Composed Of A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. It is used to supply electrical current through a redox reaction to the transfer of electrons. Use cell notation to describe galvanic cells. Describe the basic components of galvanic cells. Galvanic cells were first described in. A battery is a galvanic. A Galvanic Cell Composed Of.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary A Galvanic Cell Composed Of A battery is a galvanic cell. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. The electrochemical cell type is a galvanic cell. Use cell notation to describe galvanic cells. Use cell notation to symbolize the composition and construction of galvanic cells Describe the function of a galvanic cell and. A Galvanic Cell Composed Of.
From biochemreview.weebly.com
Galvanic cell BIOChemReview A Galvanic Cell Composed Of A battery is a galvanic cell. Describe the function of a galvanic cell and its components; The electrochemical cell type is a galvanic cell. Galvanic cells were first described in. It is used to supply electrical current through a redox reaction to the transfer of electrons. A galvanic cell is an example of how to use simple reactions between a. A Galvanic Cell Composed Of.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE A Galvanic Cell Composed Of Use cell notation to describe galvanic cells. The electrochemical cell type is a galvanic cell. A battery is a galvanic cell. Describe the function of a galvanic cell and its components; A galvanic cell converts a chemical reaction into electricity. Use cell notation to symbolize the composition and construction of galvanic cells A galvanic cell based on the spontaneous reaction. A Galvanic Cell Composed Of.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5404890 A Galvanic Cell Composed Of Galvanic cells were first described in. The electrochemical cell type is a galvanic cell. It is used to supply electrical current through a redox reaction to the transfer of electrons. Describe the function of a galvanic cell and its components; A battery is a galvanic cell. A galvanic cell is an example of how to use simple reactions between a. A Galvanic Cell Composed Of.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts A Galvanic Cell Composed Of It is used to supply electrical current through a redox reaction to the transfer of electrons. Describe the basic components of galvanic cells. Galvanic cells were first described in. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Use cell notation to symbolize the composition and construction of galvanic cells Describe the. A Galvanic Cell Composed Of.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell Composed Of The electrochemical cell type is a galvanic cell. A galvanic cell converts a chemical reaction into electricity. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Use cell notation to symbolize the composition and construction of galvanic cells Describe the function of a galvanic cell and its components; A galvanic. A Galvanic Cell Composed Of.
From www.shutterstock.com
1,233 Galvanic Cells Images, Stock Photos & Vectors Shutterstock A Galvanic Cell Composed Of Use cell notation to describe galvanic cells. A battery is a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The electrochemical cell type is a galvanic cell. Use cell notation to symbolize the composition and construction of galvanic cells Describe the function of a galvanic cell and its components;. A Galvanic Cell Composed Of.
From ar.inspiredpencil.com
Galvanic Cell Labeled A Galvanic Cell Composed Of A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. Describe the basic components of galvanic cells. Describe the function of a galvanic cell and its components; Galvanic cells were first described in. Use. A Galvanic Cell Composed Of.
From www.researchgate.net
Schematic of the galvanic cell. Download Scientific Diagram A Galvanic Cell Composed Of Use cell notation to symbolize the composition and construction of galvanic cells Describe the basic components of galvanic cells. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy. A battery is a galvanic cell. A galvanic cell converts a chemical reaction into electricity. Describe the function of a galvanic cell. A Galvanic Cell Composed Of.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 110 A Galvanic Cell Composed Of A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The electrochemical cell type is a galvanic cell. Galvanic cells were first described in. Use cell notation to symbolize the composition and construction of galvanic cells Describe the function of a galvanic cell and its components; Use cell notation to describe galvanic cells.. A Galvanic Cell Composed Of.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle A Galvanic Cell Composed Of Use cell notation to describe galvanic cells. Describe the function of a galvanic cell and its components; Galvanic cells were first described in. A galvanic cell converts a chemical reaction into electricity. The electrochemical cell type is a galvanic cell. It is used to supply electrical current through a redox reaction to the transfer of electrons. A galvanic cell is. A Galvanic Cell Composed Of.