Water Soluble Phenol Examples . If you try to dissolve more. The density of liquid phenol is around 1.07 g/cm³. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. the solubility of phenol in water is governed by the hydroxyl group present. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The solubility of phenol is due to the formation of hydrogen bonds with water. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). solubility of alcohols in water. If you try to dissolve more.
from ukmphysicalpharmacylab.blogspot.com
phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). Alcohols and water have the ability to form hydrogen bonds with one another which tends to. The density of liquid phenol is around 1.07 g/cm³. If you try to dissolve more. The solubility of phenol is due to the formation of hydrogen bonds with water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). solubility of alcohols in water. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more.
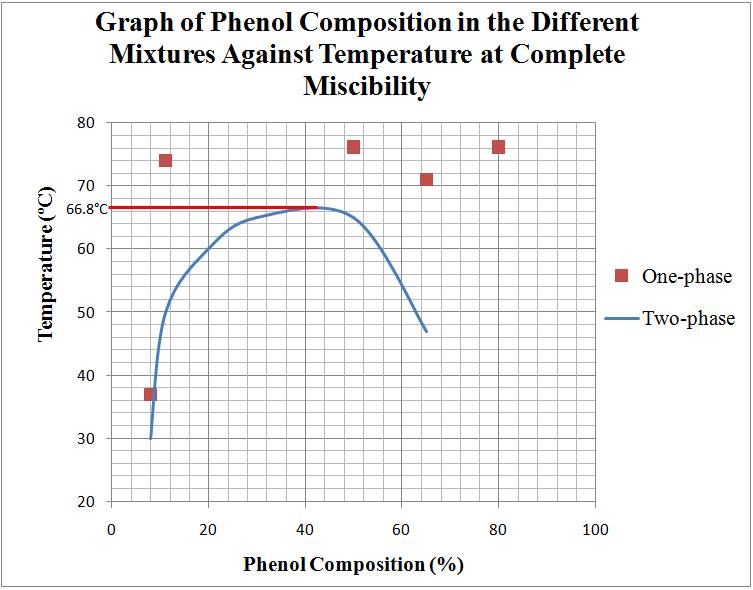
Physical Pharmacy Lab Experiment 2 Phase Diagrams (Part B) ; Mutual
Water Soluble Phenol Examples The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. If you try to dissolve more. the solubility of phenol in water is governed by the hydroxyl group present. solubility of alcohols in water. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The solubility of phenol is due to the formation of hydrogen bonds with water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. The density of liquid phenol is around 1.07 g/cm³. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen.
From mutualsolubilitycurve20163b.blogspot.com
Lab Report for Experiment 3b MUTUAL SOLUBILITY CURVE FOR PHENOL AND WATER Water Soluble Phenol Examples The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. If you try to dissolve more. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The density of liquid phenol is around 1.07 g/cm³. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). the solubility of phenol in water. Water Soluble Phenol Examples.
From chemistrypubs.com
Phenol hybridization and its solubility Chemistrupubs Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. If you try to dissolve more. If you try to dissolve more. The solubility of phenol is due to the formation of hydrogen bonds with water. Alcohols and water have the ability to form. Water Soluble Phenol Examples.
From peterkruwwatson.blogspot.com
Is Phenol Soluble in Water PeterkruwWatson Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The solubility of phenol is due to the formation of hydrogen bonds with water. the solubility of phenol in water is governed by the hydroxyl group present. Alcohols and water have the ability to. Water Soluble Phenol Examples.
From www.semanticscholar.org
Table 2 from Solubility of Phenolic Compounds in Pure Water and Water Soluble Phenol Examples Alcohols and water have the ability to form hydrogen bonds with one another which tends to. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. solubility of alcohols in water. The solubility of phenol is due to the formation of hydrogen bonds with water. If you try to dissolve more. If you try to dissolve. Water Soluble Phenol Examples.
From www.numerade.com
SOLVED ALCOHOLS, PHENOLS CARBOXYLIC ACIDS Solubility in Water (cross Water Soluble Phenol Examples Alcohols and water have the ability to form hydrogen bonds with one another which tends to. If you try to dissolve more. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). If you try to dissolve more. Phenol has a relatively high boiling point. Water Soluble Phenol Examples.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID2133789 Water Soluble Phenol Examples If you try to dissolve more. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). solubility of alcohols in water. The solubility of phenol is due to the formation of hydrogen bonds with water. The density of liquid phenol is around 1.07 g/cm³. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f).. Water Soluble Phenol Examples.
From www.bartleby.com
Answered 4.) Phenol Why slightly soluble in… bartleby Water Soluble Phenol Examples If you try to dissolve more. If you try to dissolve more. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The density of liquid phenol is around. Water Soluble Phenol Examples.
From physicalpharmacy1424.blogspot.com
Physical Pharmacy Practical Practical 2 Phase Diagram Mutual Water Soluble Phenol Examples The solubility of phenol is due to the formation of hydrogen bonds with water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). Alcohols and water have the ability to form hydrogen bonds with one another which tends to. phenols are sparingly. Water Soluble Phenol Examples.
From physicalpharmacylabreportgroupb.blogspot.com
Physical Pharmacy Lab Report Practical 2 Phase Diagrams part B Water Soluble Phenol Examples Alcohols and water have the ability to form hydrogen bonds with one another which tends to. The solubility of phenol is due to the formation of hydrogen bonds with water. If you try to dissolve more. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. Phenol has a relatively high boiling point of approximately 181.7 °c. Water Soluble Phenol Examples.
From www.slideserve.com
PPT PHENOL PowerPoint Presentation, free download ID1818685 Water Soluble Phenol Examples the solubility of phenol in water is governed by the hydroxyl group present. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. If you try to dissolve more. solubility of alcohols in water. If you try to dissolve more. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The density of. Water Soluble Phenol Examples.
From www.scribd.com
Water Phenol PDF Solubility Phase (Matter) Water Soluble Phenol Examples If you try to dissolve more. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). the solubility of phenol in water is governed by the hydroxyl group present. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The density of liquid phenol is around 1.07 g/cm³. If you try to dissolve more.. Water Soluble Phenol Examples.
From physicalpharmacylabreportgroupb.blogspot.com
Physical Pharmacy Lab Report Practical 2 Phase Diagrams part B Water Soluble Phenol Examples If you try to dissolve more. solubility of alcohols in water. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). If you try to dissolve more. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The density of liquid phenol is around 1.07 g/cm³. The hydroxyl group in phenol is involved in. Water Soluble Phenol Examples.
From kadence-yersbloghamilton.blogspot.com
Is Phenol Soluble in Water Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The solubility of phenol is due to the formation of hydrogen bonds with water. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). If you try to dissolve more. solubility of alcohols in water. The density of liquid phenol is around 1.07 g/cm³.. Water Soluble Phenol Examples.
From konishi-chem.co.jp
Watersoluble phenolic resin|R&D|KONISHI CHEMICAL IND CO.,LTD Water Soluble Phenol Examples Alcohols and water have the ability to form hydrogen bonds with one another which tends to. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). If you try to dissolve more.. Water Soluble Phenol Examples.
From ukmphysicalpharmacylab.blogspot.com
Physical Pharmacy Lab Experiment 2 Phase Diagrams (Part B) ; Mutual Water Soluble Phenol Examples Alcohols and water have the ability to form hydrogen bonds with one another which tends to. If you try to dissolve more. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. The solubility of phenol is due. Water Soluble Phenol Examples.
From dxoiubkxb.blob.core.windows.net
What Is Phenols In Drinking Water at Judy Thompson blog Water Soluble Phenol Examples phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). If you try to dissolve more. solubility of alcohols in water. The solubility of phenol is due to the formation of hydrogen bonds with water. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). the solubility of phenol in water is governed. Water Soluble Phenol Examples.
From gbu-presnenskij.ru
Water Phenol PDF Solubility Phase (Matter), 58 OFF Water Soluble Phenol Examples solubility of alcohols in water. The solubility of phenol is due to the formation of hydrogen bonds with water. If you try to dissolve more. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). the solubility of phenol in water is governed by the hydroxyl group present. Phenol has a relatively high boiling point of. Water Soluble Phenol Examples.
From ar.inspiredpencil.com
Phenol Structure Water Soluble Phenol Examples The density of liquid phenol is around 1.07 g/cm³. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). solubility of alcohols in water. the solubility of phenol in water is governed by the hydroxyl group present.. Water Soluble Phenol Examples.
From www.youtube.com
Mutual Solubility curve of Phenol and water Determination of critical Water Soluble Phenol Examples The solubility of phenol is due to the formation of hydrogen bonds with water. If you try to dissolve more. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. The hydroxyl group in phenol. Water Soluble Phenol Examples.
From www.scientific.net
Synthesis and Performances of WaterSoluble Phenolic Resin Water Soluble Phenol Examples The solubility of phenol is due to the formation of hydrogen bonds with water. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. the solubility of phenol in water is governed by the hydroxyl group present.. Water Soluble Phenol Examples.
From www.researchgate.net
Examples of phenolic compounds. Download Scientific Diagram Water Soluble Phenol Examples The solubility of phenol is due to the formation of hydrogen bonds with water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. If you try to dissolve more. solubility of alcohols in water. the solubility of phenol in water is governed by the hydroxyl group present. Phenol has a relatively. Water Soluble Phenol Examples.
From www.researchgate.net
Phase diagram of the phenol3water system under atmospheric pressure Water Soluble Phenol Examples Alcohols and water have the ability to form hydrogen bonds with one another which tends to. If you try to dissolve more. The density of liquid phenol is around 1.07 g/cm³. solubility of alcohols in water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. Phenol has a relatively high boiling point of approximately 181.7. Water Soluble Phenol Examples.
From www.researchgate.net
Chemical structure of common phenolic compounds. Download Scientific Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. The density of liquid phenol is around 1.07 g/cm³. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. If you try. Water Soluble Phenol Examples.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Water Soluble Phenol Examples the solubility of phenol in water is governed by the hydroxyl group present. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The solubility of phenol is due to the formation of hydrogen bonds with water. solubility of alcohols in water. If you try to dissolve more. If you try to dissolve more. The. Water Soluble Phenol Examples.
From www.slideserve.com
PPT Phenol PowerPoint Presentation, free download ID3560996 Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The density of liquid phenol is around 1.07 g/cm³. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The solubility of phenol is due. Water Soluble Phenol Examples.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID5469493 Water Soluble Phenol Examples If you try to dissolve more. The solubility of phenol is due to the formation of hydrogen bonds with water. If you try to dissolve more. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. the solubility of phenol in water is governed by the hydroxyl group present. The density of liquid. Water Soluble Phenol Examples.
From physicalpharmacy2013.blogspot.com
PHYSICAL PHARMACY PRACTICAL 2 Determine the solubility curve for Water Soluble Phenol Examples The density of liquid phenol is around 1.07 g/cm³. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. solubility of alcohols in water. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The solubility of phenol is due to the formation of hydrogen bonds with water. If you try to dissolve. Water Soluble Phenol Examples.
From www.youtube.com
Phase Rule Lecture 6 Phenol Water System YouTube Water Soluble Phenol Examples The density of liquid phenol is around 1.07 g/cm³. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). If you try to dissolve more. the solubility of phenol in water is governed by the hydroxyl group present. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The solubility of phenol is due. Water Soluble Phenol Examples.
From molekula.com
Purchase Phenol Red, sodium salt water soluble [34487611] online Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). The density of liquid phenol is around 1.07 g/cm³. The solubility of phenol is due to the formation of hydrogen bonds with water. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more. The hydroxyl group in. Water Soluble Phenol Examples.
From konishi-chem.co.jp
Watersoluble phenolic resin|R&D|KONISHI CHEMICAL IND CO.,LTD Water Soluble Phenol Examples Alcohols and water have the ability to form hydrogen bonds with one another which tends to. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). solubility of alcohols in water. the solubility of phenol in water is governed by the hydroxyl group present. The density of liquid phenol is around 1.07 g/cm³. If you try. Water Soluble Phenol Examples.
From www.calpaclab.com
LabChem LC182407 Phenol Red, Water Soluble, 5g, Each Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The solubility of phenol is due to the formation of hydrogen bonds with water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen. the. Water Soluble Phenol Examples.
From www.konishi-chem.co.jp
Watersoluble phenolic resin|R&D|KONISHI CHEMICAL IND CO.,LTD Water Soluble Phenol Examples If you try to dissolve more. If you try to dissolve more. The density of liquid phenol is around 1.07 g/cm³. the solubility of phenol in water is governed by the hydroxyl group present. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The solubility of phenol is due to the formation of hydrogen bonds with. Water Soluble Phenol Examples.
From eureka-patsnap-com.libproxy1.nus.edu.sg
Method for preparing clutch facing through taking water soluble phenol Water Soluble Phenol Examples The density of liquid phenol is around 1.07 g/cm³. phenols are sparingly soluble in water (9.3 gm/100gm h 2 o). The solubility of phenol is due to the formation of hydrogen bonds with water. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. the solubility of phenol in. Water Soluble Phenol Examples.
From www.researchgate.net
Content of total (a) and water soluble phenolic compounds (b Water Soluble Phenol Examples The solubility of phenol is due to the formation of hydrogen bonds with water. If you try to dissolve more. Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). solubility of alcohols in water. The density of liquid phenol is around 1.07 g/cm³. If you try to dissolve more. Alcohols and water have the ability. Water Soluble Phenol Examples.
From mutualsolubilitycurve20163b.blogspot.com
Lab Report for Experiment 3b MUTUAL SOLUBILITY CURVE FOR PHENOL AND WATER Water Soluble Phenol Examples Phenol has a relatively high boiling point of approximately 181.7 °c (359.1 °f). If you try to dissolve more. The density of liquid phenol is around 1.07 g/cm³. If you try to dissolve more. Alcohols and water have the ability to form hydrogen bonds with one another which tends to. solubility of alcohols in water. phenols are sparingly. Water Soluble Phenol Examples.