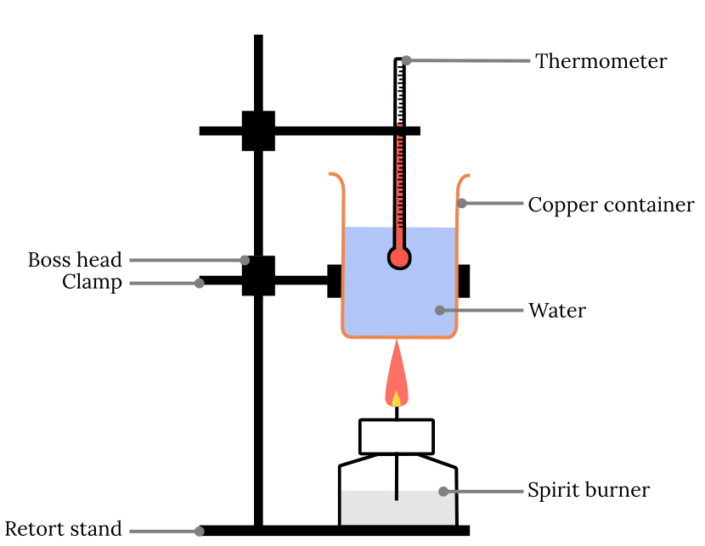
High School Chemistry Calorimetry Lab . How does the energy content in lipids and carbohydrates differ? To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). Show your work for all calculations. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. One way to do this is to place a sample of the. Calorimetry is a method used to measure the heat transfer between a system and its environment. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. Energy content is the amount of heat produced. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Chemical reactions involve the release or consumption of energy, usually in the. The specific heat of a substance is easily determined by comparing it to that of water. When 1.104 grams of iron metal are mixed with 26.023 grams of.
from www.learnable.education
In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. The specific heat of a substance is easily determined by comparing it to that of water. One way to do this is to place a sample of the. Energy content is the amount of heat produced. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. How does the energy content in lipids and carbohydrates differ? Show your work for all calculations. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). Chemical reactions involve the release or consumption of energy, usually in the. When 1.104 grams of iron metal are mixed with 26.023 grams of.
Year 11 Chemistry Practical Investigation Calorimetry Experiment
High School Chemistry Calorimetry Lab Energy content is the amount of heat produced. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. How does the energy content in lipids and carbohydrates differ? In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. The specific heat of a substance is easily determined by comparing it to that of water. Show your work for all calculations. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). When 1.104 grams of iron metal are mixed with 26.023 grams of. Calorimetry is a method used to measure the heat transfer between a system and its environment. Chemical reactions involve the release or consumption of energy, usually in the. Energy content is the amount of heat produced. One way to do this is to place a sample of the.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry High School Chemistry Calorimetry Lab If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. Show your work for all calculations. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. To remind you, endothermic reactions. High School Chemistry Calorimetry Lab.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry High School Chemistry Calorimetry Lab To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). When 1.104 grams of iron metal are mixed with 26.023 grams of. Chemical reactions involve the release or consumption of energy, usually in the. Energy content is the amount of heat produced. The specific heat of. High School Chemistry Calorimetry Lab.
From www.studocu.com
Virtual Lab Calorimetry Experiment Worksheet Virtual Lab High School Chemistry Calorimetry Lab To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). Energy content is the amount of heat produced. Calorimetry is a method used to measure the heat transfer between a system and its environment. In this lab investigation, you will use the methods of calorimetry to. High School Chemistry Calorimetry Lab.
From www.studocu.com
Calorimetry labratory 2023 0 1 Experiment 6. (1 week) (LCA High School Chemistry Calorimetry Lab How does the energy content in lipids and carbohydrates differ? One way to do this is to place a sample of the. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). Show. High School Chemistry Calorimetry Lab.
From www.studocu.com
DRY LAB A BOMB Calorimetry CHEM 1050 Studocu High School Chemistry Calorimetry Lab Show your work for all calculations. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. How does the energy content in lipids and carbohydrates differ? In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. To remind you, endothermic reactions gain. High School Chemistry Calorimetry Lab.
From www.studocu.com
Calorimetry Gizmo Student Exploration Calorimetry Lab Gizmo Warmup High School Chemistry Calorimetry Lab The specific heat of a substance is easily determined by comparing it to that of water. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. One way to do this is to. High School Chemistry Calorimetry Lab.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog High School Chemistry Calorimetry Lab One way to do this is to place a sample of the. Calorimetry is a method used to measure the heat transfer between a system and its environment. How does the energy content in lipids and carbohydrates differ? When 1.104 grams of iron metal are mixed with 26.023 grams of. Energy content is the amount of heat produced. Show your. High School Chemistry Calorimetry Lab.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment High School Chemistry Calorimetry Lab Energy content is the amount of heat produced. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Chemical reactions involve the release or consumption of energy, usually in the. Calorimetry is a method used to measure the heat transfer between a system and its. High School Chemistry Calorimetry Lab.
From www.7hills.org
Calorimetry in Chemistry Seven Hills School High School Chemistry Calorimetry Lab Calorimetry is a method used to measure the heat transfer between a system and its environment. How does the energy content in lipids and carbohydrates differ? For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. One way to do this is to place a sample of the. Chemical reactions involve the release or consumption of energy,. High School Chemistry Calorimetry Lab.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry High School Chemistry Calorimetry Lab The specific heat of a substance is easily determined by comparing it to that of water. Calorimetry is a method used to measure the heat transfer between a system and its environment. Energy content is the amount of heat produced. When 1.104 grams of iron metal are mixed with 26.023 grams of. Chemical reactions involve the release or consumption of. High School Chemistry Calorimetry Lab.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog High School Chemistry Calorimetry Lab When 1.104 grams of iron metal are mixed with 26.023 grams of. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is. High School Chemistry Calorimetry Lab.
From www.wizeprep.com
Calorimetry Wize High School Grade 12 Chemistry Textbook Wizeprep High School Chemistry Calorimetry Lab How does the energy content in lipids and carbohydrates differ? To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. One. High School Chemistry Calorimetry Lab.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL High School Chemistry Calorimetry Lab How does the energy content in lipids and carbohydrates differ? If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. Show your work for all calculations. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a. High School Chemistry Calorimetry Lab.
From www.reddit.com
[High school Calorimetry] I really need help with 3, 5 and 13 r High School Chemistry Calorimetry Lab Chemical reactions involve the release or consumption of energy, usually in the. The specific heat of a substance is easily determined by comparing it to that of water. Energy content is the amount of heat produced. Show your work for all calculations. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy. High School Chemistry Calorimetry Lab.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool High School Chemistry Calorimetry Lab Calorimetry is a method used to measure the heat transfer between a system and its environment. How does the energy content in lipids and carbohydrates differ? When 1.104 grams of iron metal are mixed with 26.023 grams of. Chemical reactions involve the release or consumption of energy, usually in the. The specific heat of a substance is easily determined by. High School Chemistry Calorimetry Lab.
From browsegrades.net
Lab Report >7.03 Calorimetry Lab Report_ Parkersburg South High School High School Chemistry Calorimetry Lab One way to do this is to place a sample of the. How does the energy content in lipids and carbohydrates differ? For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. The specific heat of a substance is easily determined by comparing it to that of water. Calorimetry is a method used to measure the heat. High School Chemistry Calorimetry Lab.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog High School Chemistry Calorimetry Lab Calorimetry is a method used to measure the heat transfer between a system and its environment. Show your work for all calculations. Energy content is the amount of heat produced. How does the energy content in lipids and carbohydrates differ? The specific heat of a substance is easily determined by comparing it to that of water. In this lab investigation,. High School Chemistry Calorimetry Lab.
From www.alamy.com
A beaker of water and a numbered test tube containing an unknown metal High School Chemistry Calorimetry Lab Energy content is the amount of heat produced. How does the energy content in lipids and carbohydrates differ? For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). The specific heat of a. High School Chemistry Calorimetry Lab.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and High School Chemistry Calorimetry Lab Energy content is the amount of heat produced. Chemical reactions involve the release or consumption of energy, usually in the. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). The specific heat of a substance is easily determined by comparing it to that of water.. High School Chemistry Calorimetry Lab.
From www.alamy.com
A digital thermometer is used to check the temperature of an unknown High School Chemistry Calorimetry Lab If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. When 1.104 grams of iron metal are mixed with 26.023 grams of. Calorimetry is a method used to measure the heat transfer between. High School Chemistry Calorimetry Lab.
From studylib.net
Chemistry 1 Unit 2 Calorimetry Lab High School Chemistry Calorimetry Lab Energy content is the amount of heat produced. The specific heat of a substance is easily determined by comparing it to that of water. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Chemical reactions involve the release or consumption of energy, usually in the. One way to do this is to place a sample of. High School Chemistry Calorimetry Lab.
From www.alamy.com
Beakers of water and a holding labeled test tubes containing unknown High School Chemistry Calorimetry Lab When 1.104 grams of iron metal are mixed with 26.023 grams of. Calorimetry is a method used to measure the heat transfer between a system and its environment. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Energy content is the amount of heat produced. The specific heat of a substance is easily determined by comparing. High School Chemistry Calorimetry Lab.
From www.studocu.com
Chemistry Calorimetry CH 1213 Studocu High School Chemistry Calorimetry Lab How does the energy content in lipids and carbohydrates differ? One way to do this is to place a sample of the. Show your work for all calculations. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. Energy content is the amount of heat produced.. High School Chemistry Calorimetry Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry High School Chemistry Calorimetry Lab To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). How does the energy content in lipids and carbohydrates differ? Chemical reactions involve the release or consumption of energy, usually in the. Calorimetry is a method used to measure the heat transfer between a system and. High School Chemistry Calorimetry Lab.
From studylib.net
Lab Calorimetry High School Science Help High School Chemistry Calorimetry Lab The specific heat of a substance is easily determined by comparing it to that of water. How does the energy content in lipids and carbohydrates differ? One way to do this is to place a sample of the. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip. High School Chemistry Calorimetry Lab.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry High School Chemistry Calorimetry Lab How does the energy content in lipids and carbohydrates differ? When 1.104 grams of iron metal are mixed with 26.023 grams of. One way to do this is to place a sample of the. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. The specific. High School Chemistry Calorimetry Lab.
From www.alamy.com
A digital thermometer is used to check the temperature of an unknown High School Chemistry Calorimetry Lab In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. The specific heat of a substance is easily determined by comparing it to that of water. One way to do this is to place a sample of the. To remind you, endothermic reactions gain energy. High School Chemistry Calorimetry Lab.
From fyogqwycu.blob.core.windows.net
Lab Calorimetry And Specific Heat Assignment Reflect On The Lab at High School Chemistry Calorimetry Lab For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. When 1.104 grams of iron metal are mixed with 26.023 grams of. One way to do this is to place a sample of the. How does the energy content in lipids and carbohydrates differ? Calorimetry is a method used to measure the heat transfer between a system. High School Chemistry Calorimetry Lab.
From www.studocu.com
Expt25 Calorimetry lab report Expt25 Calorimetry Data A High School Chemistry Calorimetry Lab If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. When 1.104 grams of iron metal are mixed with 26.023 grams of. Show your work for all calculations. One way to do this. High School Chemistry Calorimetry Lab.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu High School Chemistry Calorimetry Lab In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Calorimetry is a method used to measure the heat transfer between a system and its environment. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to. High School Chemistry Calorimetry Lab.
From saylordotorg.github.io
Calorimetry High School Chemistry Calorimetry Lab Calorimetry is a method used to measure the heat transfer between a system and its environment. How does the energy content in lipids and carbohydrates differ? To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). Chemical reactions involve the release or consumption of energy, usually. High School Chemistry Calorimetry Lab.
From printablecampusreises.z21.web.core.windows.net
How To Solve Calorimetry Problems Chemistry High School Chemistry Calorimetry Lab In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. How does the energy content in lipids and carbohydrates differ? Show your work for all calculations. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to. High School Chemistry Calorimetry Lab.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory High School Chemistry Calorimetry Lab To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). How does the energy content in lipids and carbohydrates differ? For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. The specific heat of a substance is easily determined by comparing it to. High School Chemistry Calorimetry Lab.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry High School Chemistry Calorimetry Lab Calorimetry is a method used to measure the heat transfer between a system and its environment. Chemical reactions involve the release or consumption of energy, usually in the. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). For chemical reactions, we can use calorimetry to. High School Chemistry Calorimetry Lab.
From www.wizeprep.com
Calorimetry Wize High School Grade 12 Chemistry Textbook Wizeprep High School Chemistry Calorimetry Lab To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic reactions give energy to the surroundings (δh is negative). The specific heat of a substance is easily determined by comparing it to that of water. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry is a method used to measure. High School Chemistry Calorimetry Lab.